
What’s happening?
Today, Bayer shared data from the phase 3 FINE-ONE clinical trial. These results, which were presented at the American Society of Nephrology Kidney Week in Houston, TX, showed that finerenone (Kerendia™/Firialta™) significantly reduces urine albumin-to-creatinine ratio (UACR), a measure of kidney damage, in people with chronic kidney disease (CKD) associated with type 1 diabetes (T1D). This is an exciting win for the T1D community!

Finerenone
Finerenone is a drug that has already been approved for the treatment of CKD in type 2 diabetes. In CKD, the hormone aldolsterone is overactive, leading to kidney damage. Finerenone blocks this hormone’s activity to protect the kidneys from further damage.
What did the FINE-ONE study show?
The global, phase 3 FINE-ONE clinical trial investigated the use of finerenone in people with CKD associated with T1D. This trial achieved its primary outcome: finerenone significantly reduced UACR—a measure of kidney damage—compared to placebo.
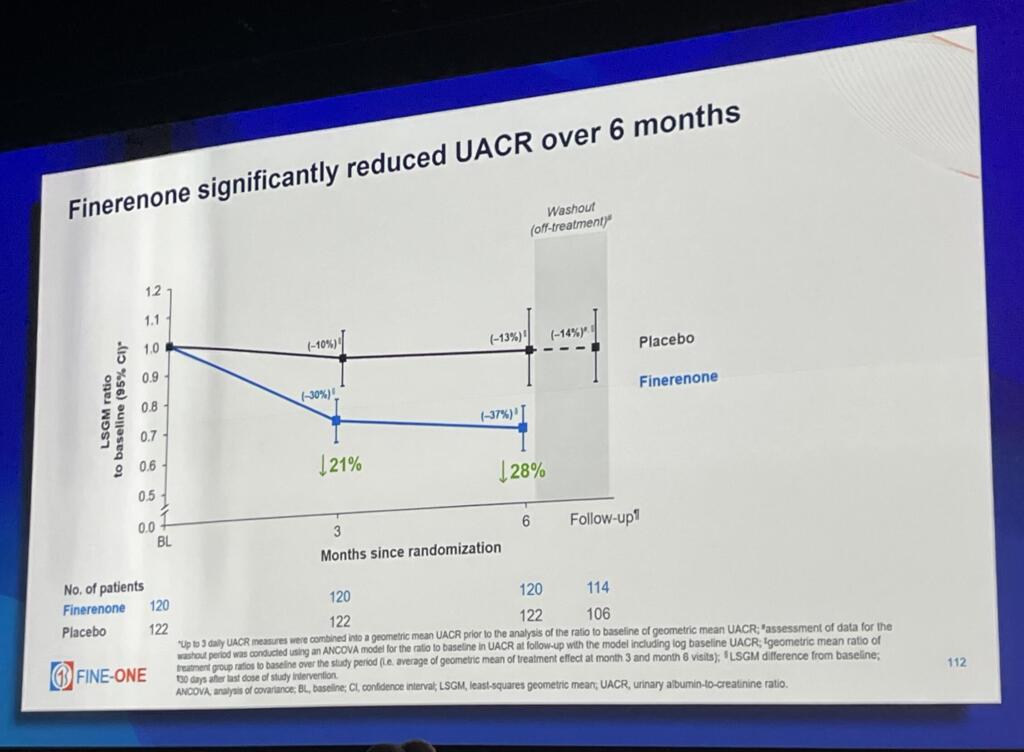
So, what does this mean? Previous clinical trials with people who have type 2 diabetes and CKD have shown that reductions in UACR from finerenone are associated with positive long-term health outcomes. In people with T1D, reductions in UACR caused by finerenone are likely indicative of lower risk of kidney disease progression, kidney failure, and cardiovascular events.
Finerenone was well-tolerated, with no new safety issues reported and few serious adverse events. Based on these results, Bayer intends to submit the data for regulatory review, with the goal of expanding finerenone’s indication to include treatment of CKD in people living with T1D. If finerenone gets approved, this would be an incredible step forward for people living with the burden of both kidney disease and T1D.
Why does this matter for T1D?
CKD is one of the most common complications of T1D. Nearly a third of people living with T1D will develop CKD, increasing the risk of both kidney failure and cardiovascular disease. Right now, the treatment options for people with T1D and CKD are limited—and there is a major need for therapies that can address this.
Finerenone is the first therapy in three decades to achieve positive outcomes for CKD in people with T1D. This is a huge step forward in the right direction, and this exciting data has the potential to transform the lives of people living with T1D with CKD if finerenone gets regulatory approval.
The bigger picture
Breakthrough T1D strategically collaborated with Bayer to support the FINE-ONE clinical trial, and we are committed to further collaboration with Bayer to advance therapies for people with T1D. Working together with Bayer and other companies will allow us to deliver more treatments to people with T1D to address complications like kidney disease.

“People with type 1 diabetes and chronic kidney disease face an immense burden due to their increased risk for both kidney and cardiovascular events,” said Jonathan Rosen, Ph.D., Research Director at Breakthrough T1D. “Breakthrough T1D remains committed to collaborating with Bayer to improve kidney care for people with type 1 diabetes.”
As a part of our Improving Lives portfolio, we aim to identify and support treatments and therapies that can address complications that arise from T1D, like CKD. The FINE-ONE trial opens the door for more therapies to come that could transform the landscape of available therapies for people with T1D, and industry investment in this space helps drive progress forward faster than ever. The T1D community needs more options, and our strategic industry partnerships will help make this possible.
We look forward to seeing the regulatory decisions that stem from FINE-ONE, and we are incredibly excited for what the future holds for CKD and T1D.