Our history
Since our founding in 1970, Breakthrough T1D has played a critical role in every major type 1 diabetes milestone.
A timeline of our key accomplishments
1970s

Founding of Juvenile Diabetes Foundation
The Juvenile Diabetes Foundation, now Breakthrough T1D, is founded by Lee Ducat, Carol Lurie, Erwin Lurie, and a group of parents whose children have T1D. Their conviction is clear: through research, T1D can and will be cured.
Read More About Where Our Journey BeganCreation of hemoglobin A1c (HbA1c) test
Breakthrough T1D-funded researcher Anthony Cerami, Ph.D., demonstrates that hemoglobin can be used to more effectively measure blood glucose level management. This discovery led to the creation of the hemoglobin A1c (HbA1c) test which is now the gold standard for approval of diabetes drugs.
Read More About How HbA1c Came To Be1980s
Genetically engineered human insulin created
Breakthrough T1D-funded science leads to the development of genetically engineered insulin—the first human protein to be cloned and made by genetic engineering. It is marketed under the name Humulin and receives FDA approval.
Read More About The Development Of Humulin
First commercially available insulin pump
The first insulin pump is developed and commercialized by Medtronic, one of Breakthrough T1D’s industry partners.
Learn More About Insulin PumpsFirst islet transplant in humans
Breakthrough T1D-backed researcher Paul Lacey, M.D., Ph.D., performs the first islet transplant in humans.
Explore Our Research In Cell Therapies1990s
Connection established between high blood sugar levels and diabetic eye disease
Breakthrough T1D research establishes the causal relationship between diabetic eye disease and high blood sugar levels.
Read More About Our Research In Diabetic Eye Disease
Creation of Special Diabetes Program
Congress creates the Special Diabetes Program (SDP) to address the limitations in diabetes research due to a lack of funding. The SDP has been pivotal to therapies currently making life better for those with T1D and is funding research for future cures. As of April 2024, the SDP has dedicated more than $3.5 billion to T1D research.
Learn More About The Special Diabetes Program
First Children's Congress
Led by Mary Tyler Moore, the first Breakthrough T1D Children’s Congress (formerly JDRF Children’s Congress) empowers children with T1D to share their experiences with Members of Congress and advocate for Federal funding of T1D research.
Learn More About Children's Congress2000s
Edmonton Protocol developed
Breakthrough T1D researchers develop the Edmonton Protocol for islet transplants, which greatly improves the success rate for transplanting insulin-producing cells in people with T1D.
Read More About The Edmonton Protocol
Artificial Pancreas Consortium
Breakthrough T1D launches the Artificial Pancreas Consortium, bringing together the best scientists from the public, private, and academic sectors to work collaboratively on the development and delivery of artificial pancreas (AP) systems.
Network for Pancreatic Organ Donors with Diabetes launches
Breakthrough T1D launches the Network for Pancreatic Organ Donors with Diabetes (nPOD), the world’s only tissue bank for donor pancreases and related tissues from people with and at risk for T1D. Research from this initiative has fundamentally changed our understanding of T1D.
Learn More About nPOD
Broader CGM use
A Breakthrough T1D-funded clinical trial demonstrates efficacy of continuous glucose monitors (CGMs) in helping to manage blood sugar, with lower HbA1c levels and reduced rates of severe hypoglycemia. This leads to CGM-payer coverage for these life-changing devices.
Learn More About Continuous Glucose Monitors2010s
FDA approval of Lucentis for the treatment of diabetic macular edema
Breakthrough T1D researchers show in clinical trials that a drug called Lucentis is effective against diabetic eye disease. It receives FDA approval, making it the first drug to be approved for diabetic eye disease and the first new treatment in 25 years.
FDA releases AP systems guidance
The FDA releases its final AP systems guidance, based on recommendations from Breakthrough T1D, providing a clear and reasonable regulatory roadmap.

Stem cells converted into insulin-producing beta cells
Doug Melton, Ph.D., a Breakthrough T1D-backed researcher, develops an innovative protocol for rapidly converting human stem cells into insulin-producing beta cells in the lab, significantly speeding up the conversion process.
Learn More About Our Research In Cell TherapiesFirst clinical trial in cell replacement therapy
With Breakthrough T1D support, ViaCyte initiates the first clinical trial testing a stem-cell-derived beta cell replacement therapy for T1D. Initial results showed that implanted cells can produce insulin.
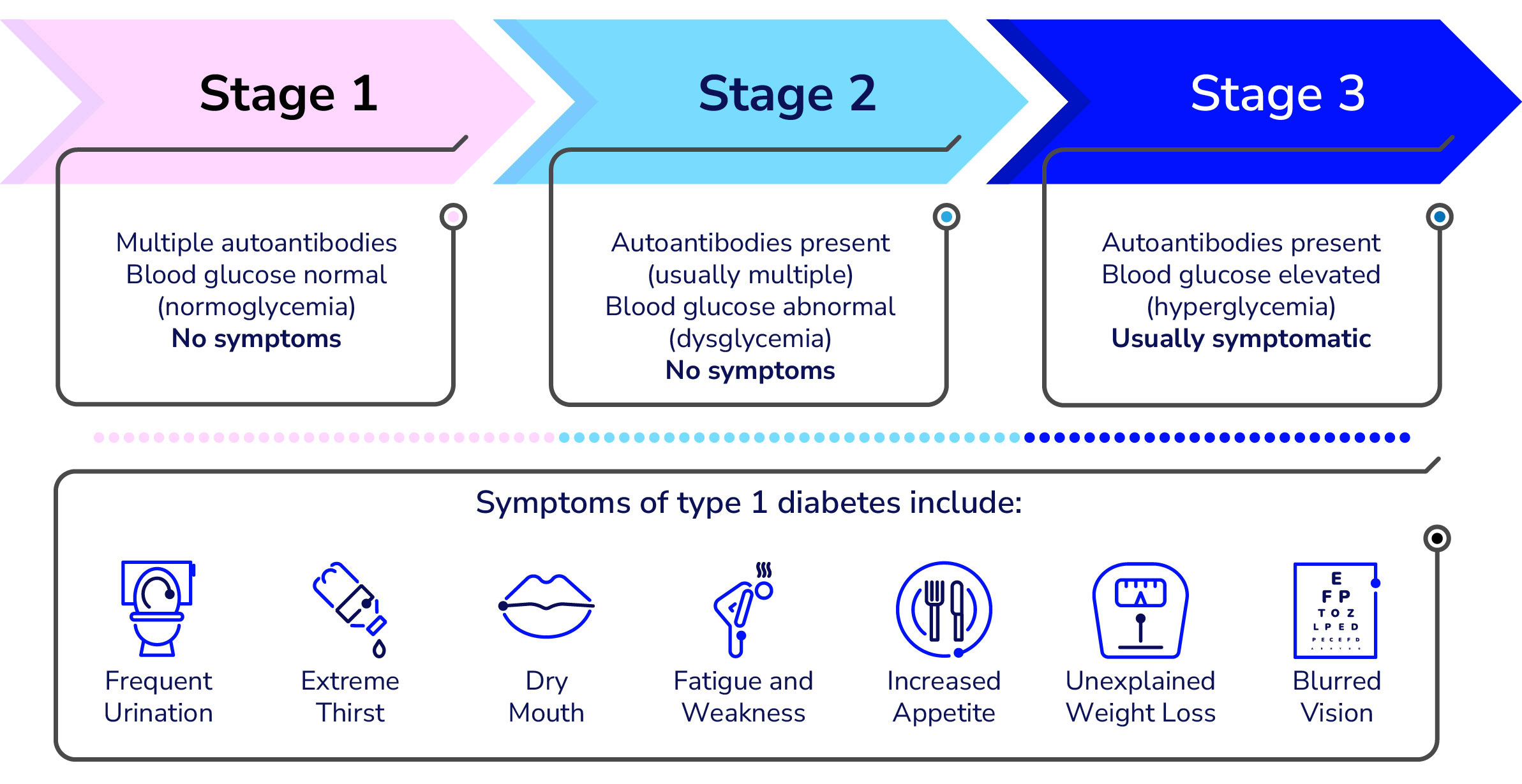
Development of T1D Staging Classification system
Breakthrough T1D leads the effort for the development of a Staging Classification system that characterizes the earliest stages of T1D which enables more precise monitoring of disease progression and better design of clinical trials aimed at preventing the disease.
Launch of The T1D Fund: A Breakthrough T1D Venture
Breakthrough T1D volunteers eager to catalyze more private investment in T1D cure therapies launch the world’s first venture philanthropy fund dedicated to type 1 diabetes—the T1D Fund: A Breakthrough T1D Venture. The T1D Fund has transformed the movement to cure T1D by creating an investment market that has attracted more than $800 million in private venture capital.
Learn More About The T1D Fund
First artificial pancreas system approved by FDA
The FDA approves Breakthrough T1D industry partner Medtronic’s MiniMed 670G hybrid closed-loop AP system, the first ever approved to automate insulin dosing to reduce high blood sugar levels. Breakthrough T1D played an integral role at all stages, funding critical research and working with the FDA to establish a regulatory framework.
Learn More About Automated Insulin Delivery Systems
Study shows CGM use in pregnancy improves health outcomes for mothers and babies
Results are published from the Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial, a trial co-funded by Breakthrough T1D and the Canadian Institutes of Health Research (CIHR). The results show that CGM use during and before pregnancy improves the health outcomes for both mothers and babies while reducing costs for neonatal hospitalization.
Learn More About Pregnancy and T1DStudy shows blood pressure medication can preserve insulin production in newly diagnosed
In a Breakthrough T1D-funded clinical trial, Verapamil, a widely-used blood pressure medication, is shown to preserve insulin production in adults with recent-onset type 1 diabetes by preserving beta cell function.
Read More About This StudyDisease-modifying therapy delays onset of T1D
Teplizumab, an anti-CD3 monoclonal antibody, is shown to delay the development of T1D in at-risk individuals by an average of two years.
Learn More About Teplizumab2020s
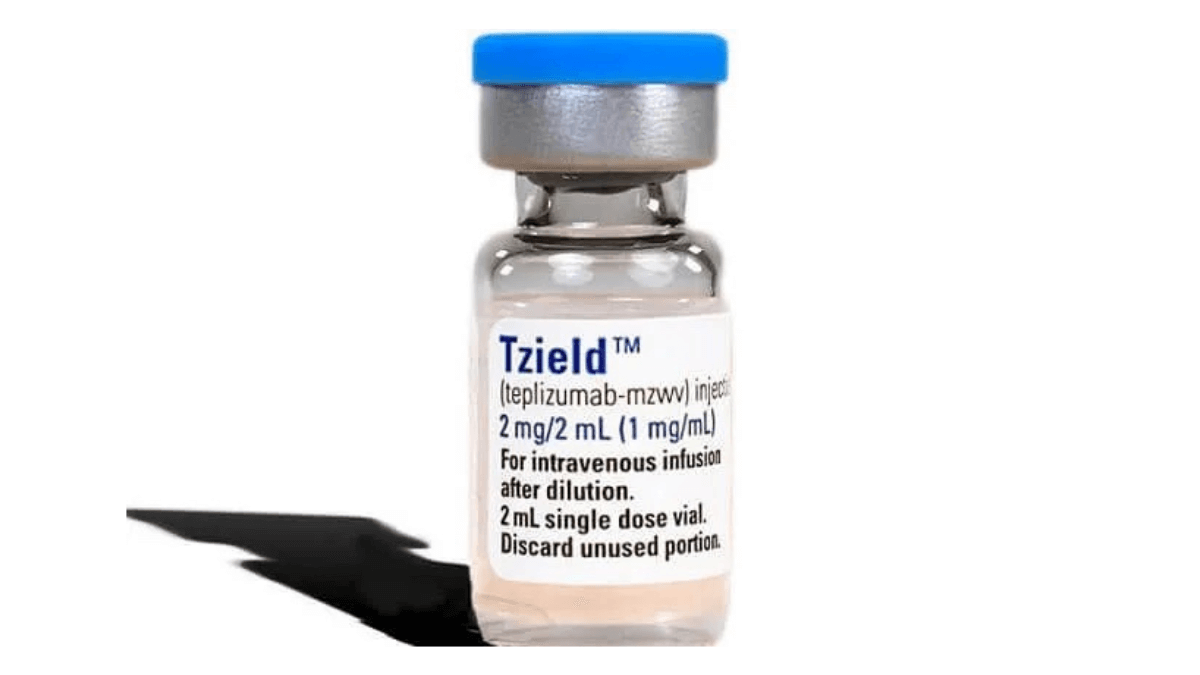
First disease-modifying therapy for T1D approved by FDA
The FDA approves Tzield™ (teplizumab-mzwv), the first disease-modifying therapy (a therapy that can slow, halt, or reverse the progression of a disease), for individuals at-risk for developing T1D. This watershed moment for the T1D community has been supported by Breakthrough T1D for decades.
Read More About This Groundbreaking TherapyA plan to produce affordable insulin
With Breakthrough T1D support, nonprofit pharmaceutical manufacturer Civica announces plans to produce lower cost biosimilar insulins. These insulins will be available to anyone, regardless of insurance status, at no more than $30/vial or $55/box of five pens.
Learn More About Our Partnership With Civica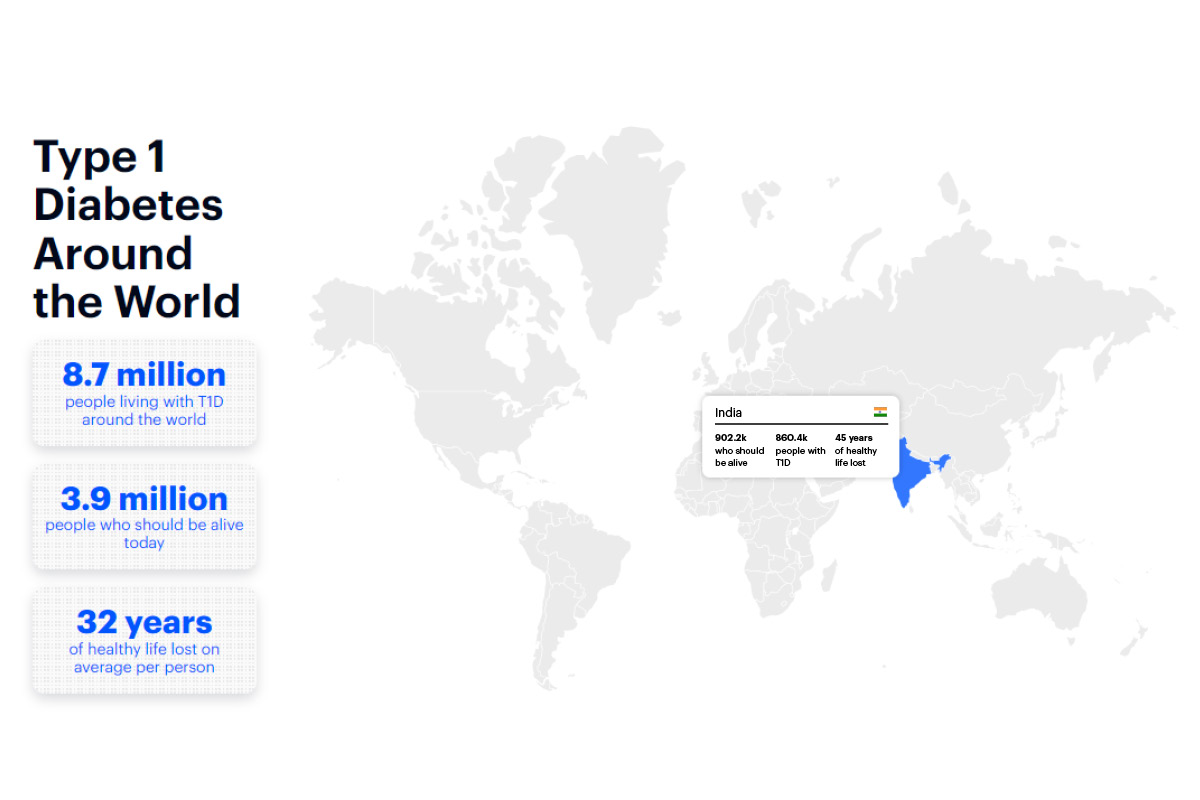
Launch of the T1D Index
The T1D Index, an initiative led by Breakthrough T1D, provides the first-ever comprehensive picture of T1D. The index's data shows the presence and growth of T1D in every country around the globe. The index inspires action by providing a rare perspective into the data behind T1D and the strategies that could save countless lives while further accelerating cures.
Read More About The T1D Index, A First-of-its-Kind Lifesaving ToolAutoantibodies recognized as indicator of T1D risk
Regulators acknowledge that markers in the blood, called autoantibodies, could be used to screen for risk of developing T1D—an effort that Breakthrough T1D championed for decades to improve chances of identifying risk status and reducing dangerous conditions like DKA often experienced at T1D diagnosis.
Learn More About Early Detection
FDA approves first cell replacement therapy
The FDA approves Lantidra™, the first beta cell replacement therapy. This establishes a key regulatory pathway Breakthrough T1D is working to accelerate for stem-cell derived based therapies, which do not rely on a limited supply of deceased donor islets.
Explore About Our Research In Cell Therapies
AP systems improve pregnancy outcomes
A Breakthrough T1D-funded study finds AP systems substantially reduce maternal blood sugars, benefiting mothers and babies. The study, published in the New England Journal of Medicine, recommends a key advance in T1D standards of care: that all pregnant women with T1D have access to AP systems.
Learn More About Pregnancy and T1DDisease-modifying therapies benefit newly diagnosed
Breakthrough T1D-supported clinical trials show that three different disease-modifying therapies preserve beta cell function and slow progression of new-onset T1D. Translation to clinical use could be streamlined as all three medications are already approved for other conditions or for delaying onset of T1D.
Explore Our Funded Research In Disease-Modifying Therapies
We are Breakthrough T1D
We discovered that we needed a name that reflected who we are—and where we’re going. It was time to carry the momentum created by more than 50 years of research, advocacy, and community into a brand that reflected who we are, where we’ve come from, and where we’re going. We are now Breakthrough T1D.
Learn More About Our New BrandBreakthrough T1D spearheads the formation of monitoring guidelines
Breakthrough T1D spearheaded an effort to develop the first internationally agreed-upon guidance for monitoring children, adolescents, and adults who test positive for T1D autoantibodies, along with recommended monitoring frequencies and actions for healthcare professionals when the risk of progression toward symptomatic T1D is high.
Read More About Monitoring Guidelines.
Project ACT launches
Cell therapies could cure people with T1D. Project ACT is a Breakthrough T1D initiative to dramatically speed cell therapy products as T1D cures through coordinated efforts to simultaneously advance research, development, regulatory, access, and adoption.
Learn More About Project ACT.Vertex launches pivotal trial for stem-cell derived islet therapy
Vertex's Phase 1/2 trial for VX-880, a stem cell-derived islet therapy, converted to a Phase 3 pivotal trial, enrolling 50 total people. It’s the first time a scalable cure for some people with type 1 diabetes entered a Phase 3 clinical trial.
Learn More About This Pivotal Trial.
Cell therapy first: transplanted islets working without immunosuppressives
Sana Biotechnology, supported by the T1D Fund: A Breakthrough T1D Venture released significant clinical data: The first person with type 1 diabetes who received deceased donor islets engineered to evade the immune system is producing insulin without immunosuppression.
Read More About Sana's Study.
Breakthrough T1D Medical Affairs unit created
Closing the gap between access to and adoption of T1D therapies is a mission priority for Breakthrough T1D. We announced the establishment of a Medical Affairs unit to address the numerous challenges contributing to the slow adoption of groundbreaking T1D therapies.
Read More About Our Medical Affairs Unit.