2025 Top T1D advances: Full speed ahead
How quickly a year goes by—and a lot has happened for type 1 diabetes (T1D) in 2025! In these last few weeks leading up to the new year, Breakthrough T1D would like to take a moment to reflect on how far we’ve come in the past 365 days. Read on for the greatest T1D accomplishments […]

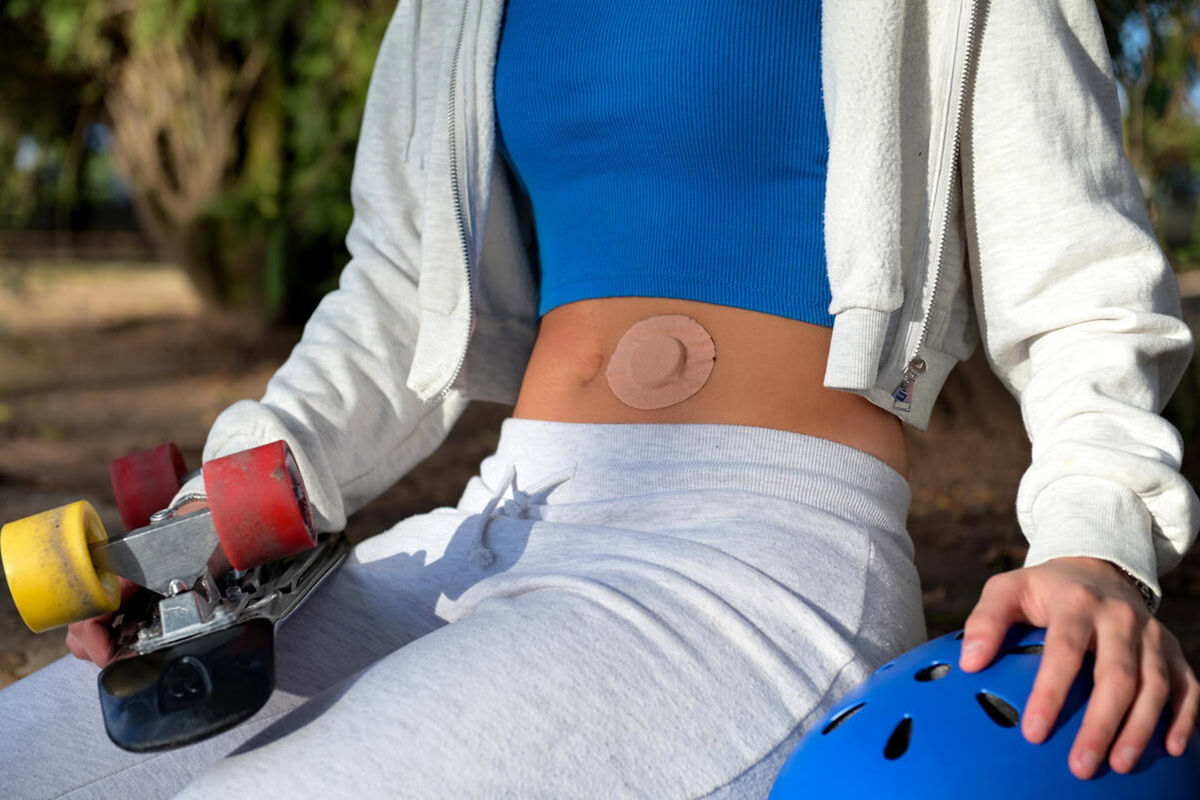
Smart devices, angry skin: The underside of diabetes tech
Skin reactions from adhesives on insulin pumps and CGMs are common. Learn why they occur and how to reduce irritation.
See Medical Affairs. See T1D.
Closing the gap between access to and adoption of type 1 diabetes therapies is a mission priority. Chris Dunn represents these efforts in action.

One man, seven marathons, seven continents, seven days
Forrest Johnson embarked on a globe-spanning, 184-mile sprint with The Great World Race to fundraise for type 1 diabetes research.

See our advocates. See T1D.
Through grassroots support, Breakthrough T1D advocates help advance treatments, influence policy, and improve global access to care.

Cardiovascular disease and T1D: Advancing breakthroughs in Europe and beyond
What’s happening? Today, Breakthrough T1D co-hosted an event with leading international diabetes organizations to discuss cardiovascular disease (CVD) and type 1 diabetes (T1D)—where we are, challenges that remain, and how we can work together in Europe and beyond to address this need for the T1D community. The focus of the event: Doing more for CVD […]

Highlights from the 2025 ISPAD conference
Bonjour from Montreal, Canada, where the latest and greatest in type 1 diabetes (T1D) research was presented at the International Society for Pediatric and Adolescent Diabetes (ISPAD) 51st Annual Conference. ISPAD is a yearly highlight of the T1D conference calendar, and this year was no exception. Scientists, clinicians, researchers, industry members, people with diabetes, and […]
Demystifying the pipeline of T1D therapies and devices: Turning ideas into reality
What is the pipeline? Every new medical device, therapy, treatment, and drug—including those for type 1 diabetes (T1D)—goes through the drug development pipeline. Getting a new therapy or device from the earliest stages of research eventually into the hands of people with T1D is a complicated process. Science takes time (from years to decades!), money […]

See our cures research. See T1D.
There are three ways we can get to cures for type 1 diabetes faster: cell therapies, early detection, and disease-modifying therapies.

Finerenone shows positive results for chronic kidney disease associated with T1D
What’s happening? Today, Bayer shared data from the phase 3 FINE-ONE clinical trial. These results, which were presented at the American Society of Nephrology Kidney Week in Houston, TX, showed that finerenone (Kerendia™/Firialta™) significantly reduces urine albumin-to-creatinine ratio (UACR), a measure of kidney damage, in people with chronic kidney disease (CKD) associated with type 1 […]