
2025 Top T1D advances: Full speed ahead
How quickly a year goes by—and a lot has happened for type 1 diabetes (T1D) in 2025! In these last few weeks leading up to the new year, Breakthrough T1D would like to take a moment to reflect on how far we’ve come in the past 365 days. Read on for the greatest T1D accomplishments […]

See our advocates. See T1D.
Through grassroots support, Breakthrough T1D advocates help advance treatments, influence policy, and improve global access to care.
Demystifying the pipeline of T1D therapies and devices: Turning ideas into reality
What is the pipeline? Every new medical device, therapy, treatment, and drug—including those for type 1 diabetes (T1D)—goes through the drug development pipeline. Getting a new therapy or device from the earliest stages of research eventually into the hands of people with T1D is a complicated process. Science takes time (from years to decades!), money […]
Tzield receives voucher for expedited review in stage 3 T1D
This past week, the FDA and Sanofi, the maker of Tzield, made an important announcement. Tzield, the first disease-modifying therapy approved for delaying onset of stage 3 type 1 diabetes (T1D) in people with stage 2 T1D, has been accepted into the FDA Commissioner’s National Priority Voucher (CNPV) program for people with stage 3 T1D. […]
Civica long-acting insulin available on January 1, 2026
Nearly 3 years ago, Breakthrough T1D joined forces with Civica Rx, a non-profit pharmaceutical company, to manufacture and distribute low-cost insulins. Starting on January 1, 2026, the first of those insulins, insulin glargine-yfgn (a long-acting insulin interchangeable with Lantus®), will be available for purchase. 5 Pens, No More Than $55 – for anyone While we […]

Special Diabetes Program yields $50+ billion in Federal healthcare savings
Analysis compiled by Avalere Health and supported by Breakthrough T1D (formerly JDRF) finds that research funded by the Special Diabetes Program (SDP) has yielded more than $50 billion in federal healthcare savings. Created by Congress in 1997 and administered by the National Institutes of Health (NIH), the SDP provides federal funding to support research to prevent, treat, and […]

Study: Patients willing to incur risks for benefits of novel T1D therapies
A new Breakthrough T1D-funded study finds that people with T1D and their caregivers are largely willing to try advanced new T1D therapies.

A new Breakthrough T1D publication is paving the way for beta cell replacement therapies
Breakthrough T1D’s newest publication outlines a roadmap for beta cell replacement therapies for type 1 diabetes.
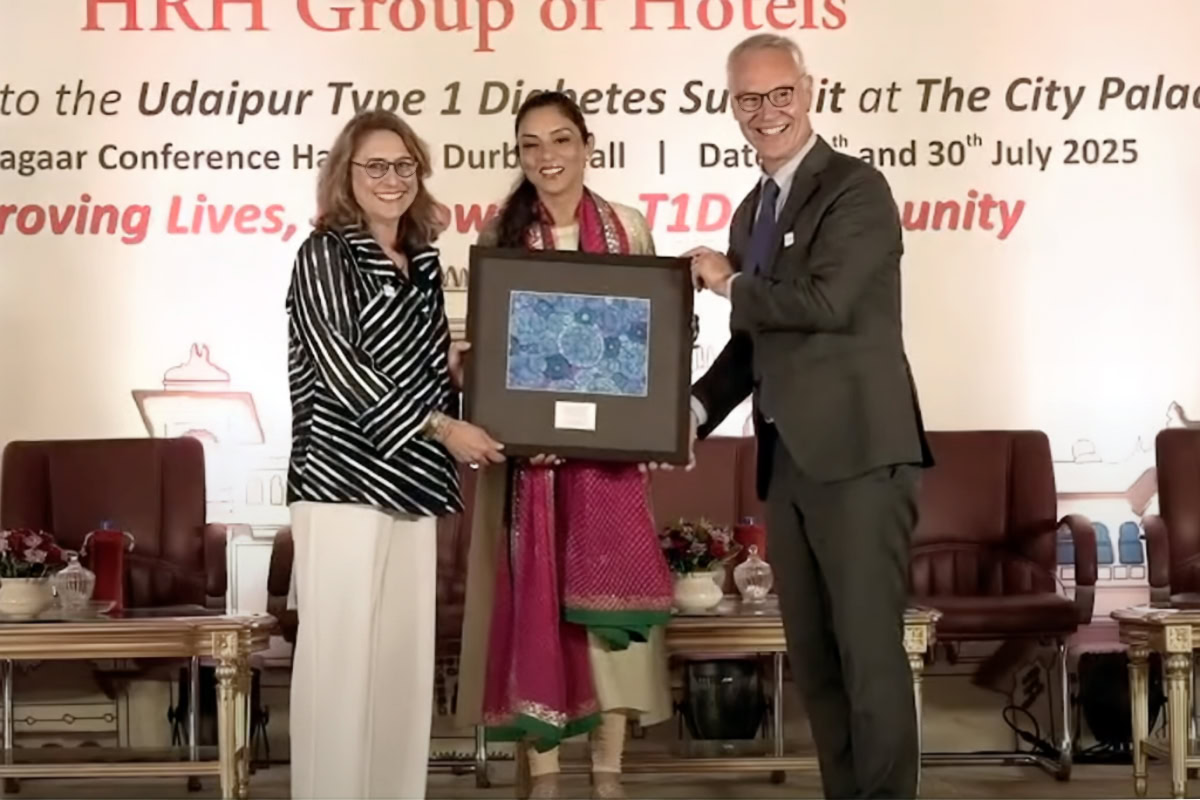
Advancing access, equity, and action in India and around the world
The Udaipur Type 1 Diabetes Summit gathered international leaders to develop a collaborative roadmap for strengthening T1D care across India.

“We are our own solution:” A T1D parent’s reflection on progress
Moira McCarthy reflects on the transformative impact of automated insulin delivery systems, and how the T1D community made it possible.