See Medical Affairs. See T1D.
Closing the gap between access to and adoption of type 1 diabetes therapies is a mission priority. Chris Dunn represents these efforts in action.

Two new trials investigating baricitinib to delay T1D
What’s happening? Earlier today, Eli Lilly and Company announced that they are launching two new clinical trials for baricitinib in type 1 diabetes (T1D). These phase 3 trials will investigate whether the drug can delay T1D onset or progression and will open for recruitment soon. Read on to learn more about the trials, why this […]
Tzield receives voucher for expedited review in stage 3 T1D
This past week, the FDA and Sanofi, the maker of Tzield, made an important announcement. Tzield, the first disease-modifying therapy approved for delaying onset of stage 3 type 1 diabetes (T1D) in people with stage 2 T1D, has been accepted into the FDA Commissioner’s National Priority Voucher (CNPV) program for people with stage 3 T1D. […]
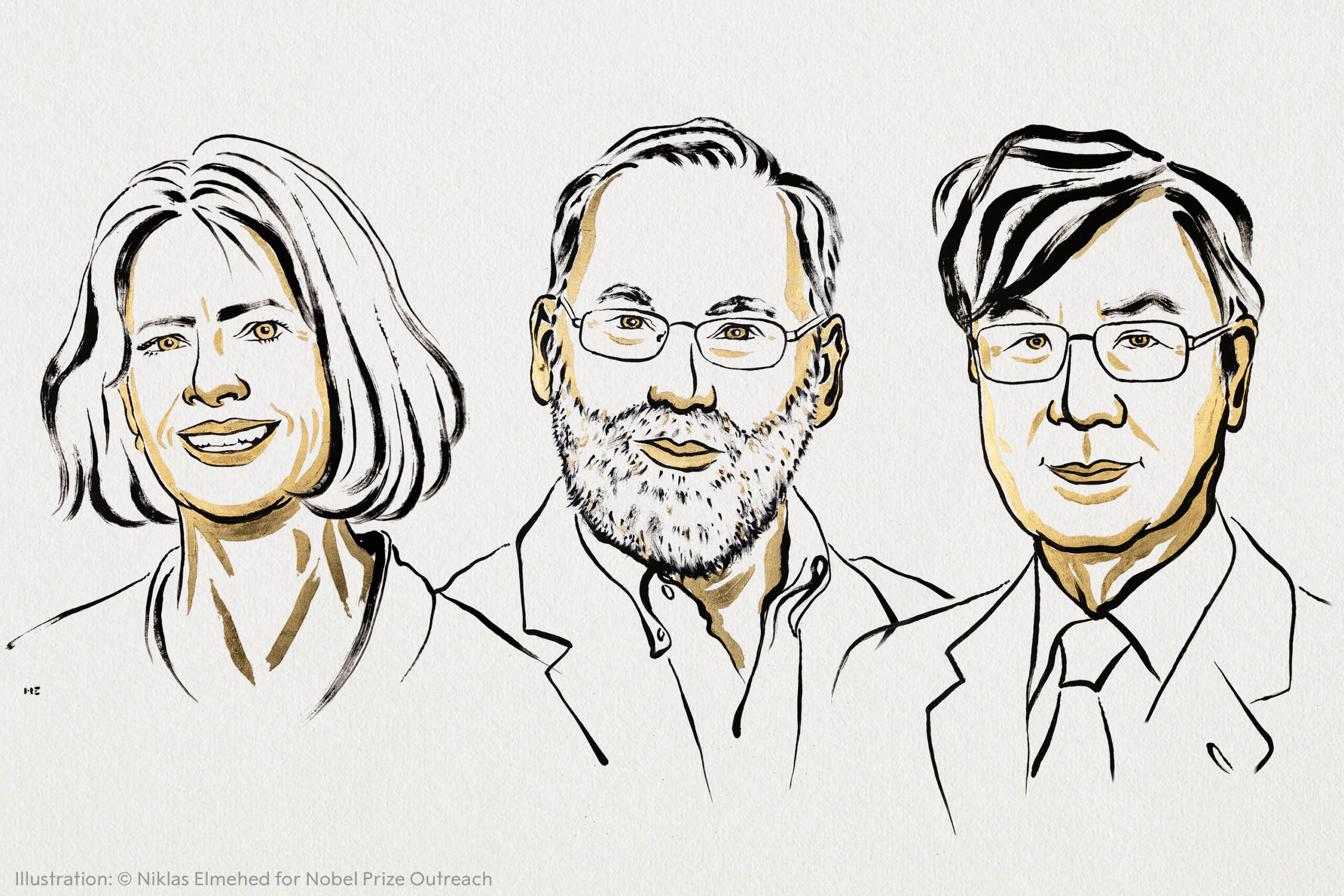
The Nobel Prize goes to… Immunologists (with a Breakthrough T1D connection!)
The Nobel Prize in Physiology or Medicine went to three immunologists, including one, Fred Ramsdell, who founded a T1D Fund company.

Study: Patients willing to incur risks for benefits of novel T1D therapies
A new Breakthrough T1D-funded study finds that people with T1D and their caregivers are largely willing to try advanced new T1D therapies.

Who is at risk for type 1 diabetes?
Although its exact causes are unknown, researchers have uncovered type 1 diabetes risk factors that increase a person's likelihood of developing the condition.

ADA 2025 Recap: Cures
Breakthrough T1D was in Chicago, IL for ADA 2025. Here we report on the latest advancements in cures for type 1 diabetes.

From unexpected diagnosis to DIAGNODE-3 participant
Katie Howell was diagnosed with type 1 diabetes last year at age 25. Now, she's the first DIAGNODE-3 trial participant in NYC.

Medical Affairs: Closing the gap between access and adoption
Breakthrough T1D's new Medical Affairs unit will address the numerous challenges contributing to the slow adoption of groundbreaking T1D therapies.

A behind-the-scenes look at DIAGNODE-3: Phase 3 clinical trials of Diamyd® for T1D
DIAGNODE-3 is a phase 3 clinical trial of the disease-modifying therapy Diamyd® that aims to preserve insulin-producing beta cells.