
Spotlight on OPF-310: A porcine-derived cell therapy in clinical trials
One of our most promising avenues toward cures for type 1 diabetes (T1D) is cell therapies. Cell therapies replace destroyed beta cells with functional, insulin-producing cells to restore insulin therapy independence. Right now, there are nearly a dozen cell therapies in clinical testing—and OPF-310 is one of them. OPF-310 cells are derived from porcine (pig) […]
Navigating issues with your continuous glucose monitor (CGM)
Breakthrough T1D created a guide for sensor issues, from what to do if symptoms and CGM reading do not match to how to get a replacement.

Hot off the press: Continuous ketone monitoring guidelines are here
What’s happening? Continuous ketone monitoring (CKM) is on its way. A new paper in The Lancet Diabetes & Endocrinology, titled “International expert recommendations on the application and utility of continuous ketone monitoring for people with diabetes,” was published by Breakthrough T1D and expert collaborators, spearheaded by our Chief Medical Officer, International, Thomas Danne, M.D., Ph.D. […]
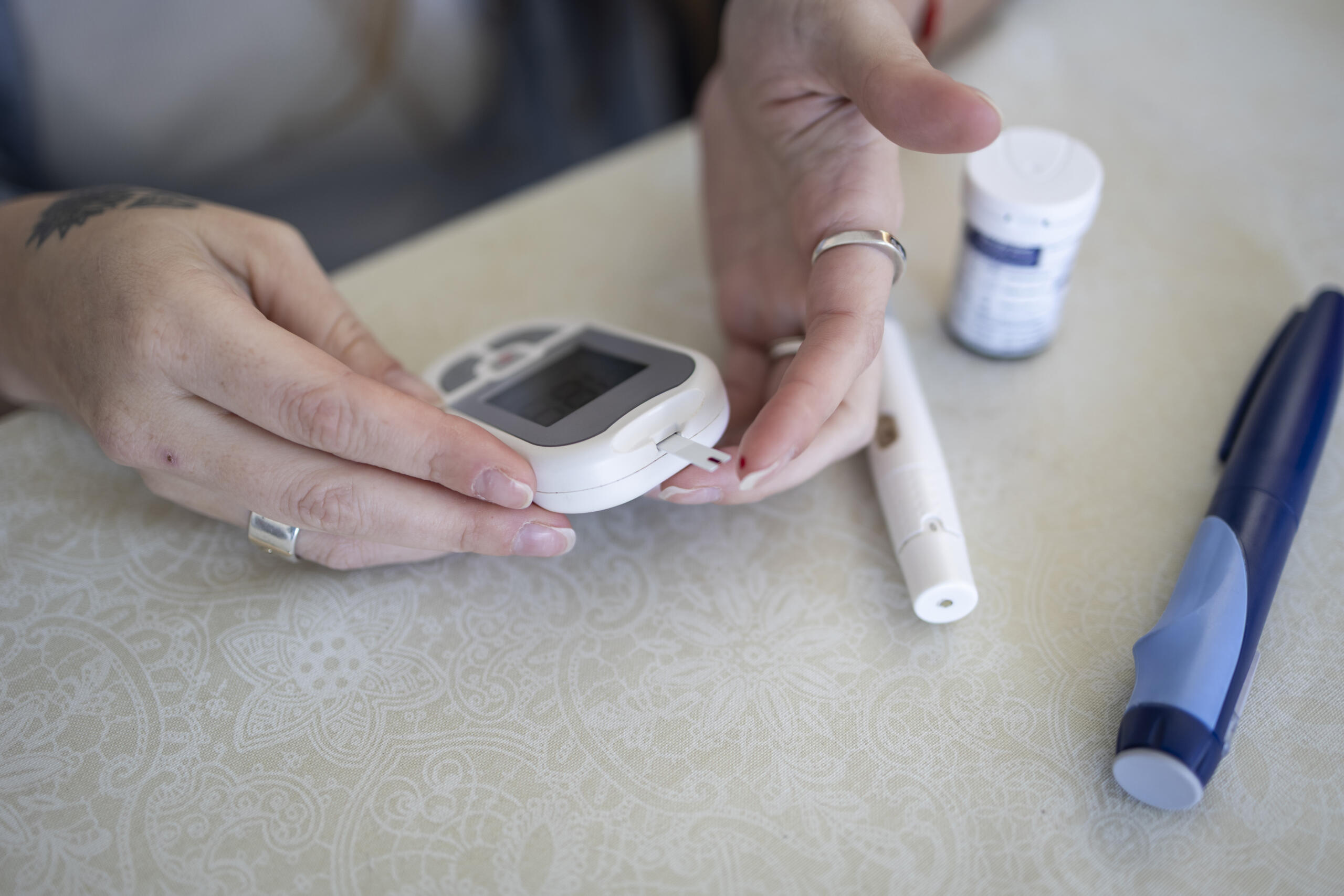
Support and resources for your CGM
Support and resources for issues with continuous glucose monitors (CGMs), including inaccurate readings and faulty sensors.

2025 Top T1D advances: Full speed ahead
How quickly a year goes by—and a lot has happened for type 1 diabetes (T1D) in 2025! In these last few weeks leading up to the new year, Breakthrough T1D would like to take a moment to reflect on how far we’ve come in the past 365 days. Read on for the greatest T1D accomplishments […]
See Medical Affairs. See T1D.
Closing the gap between access to and adoption of type 1 diabetes therapies is a mission priority. Chris Dunn represents these efforts in action.

Cardiovascular disease and T1D: Advancing breakthroughs in Europe and beyond
What’s happening? Today, Breakthrough T1D co-hosted an event with leading international diabetes organizations to discuss cardiovascular disease (CVD) and type 1 diabetes (T1D)—where we are, challenges that remain, and how we can work together in Europe and beyond to address this need for the T1D community. The focus of the event: Doing more for CVD […]

Highlights from the 2025 ISPAD conference
Bonjour from Montreal, Canada, where the latest and greatest in type 1 diabetes (T1D) research was presented at the International Society for Pediatric and Adolescent Diabetes (ISPAD) 51st Annual Conference. ISPAD is a yearly highlight of the T1D conference calendar, and this year was no exception. Scientists, clinicians, researchers, industry members, people with diabetes, and […]
Demystifying the pipeline of T1D therapies and devices: Turning ideas into reality
What is the pipeline? Every new medical device, therapy, treatment, and drug—including those for type 1 diabetes (T1D)—goes through the drug development pipeline. Getting a new therapy or device from the earliest stages of research eventually into the hands of people with T1D is a complicated process. Science takes time (from years to decades!), money […]

See our cures research. See T1D.
There are three ways we can get to cures for type 1 diabetes faster: cell therapies, early detection, and disease-modifying therapies.