
Top Type 1 Diabetes Advances of Fiscal Year 2023
Breakthrough T1D highlights the top type 1 diabetes advances we've seen during fiscal year 2023, including cures and life-improving therapies.

A Bittersweet Goodbye for Breakthrough T1D Champion Cynthia Rice
JDRF’s Chief Mission Strategy Officer will leave behind quite a legacy once she steps down from her role at Breakthrough T1D at the end of March 2023.
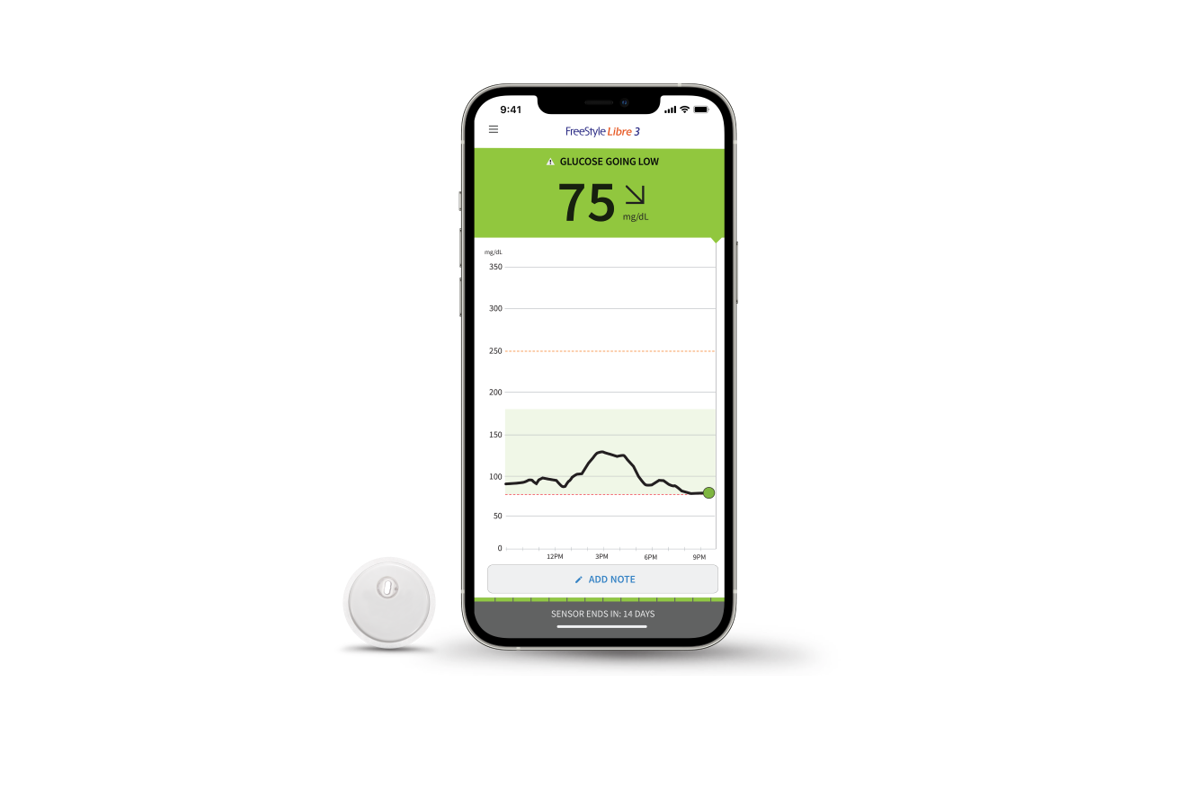
FDA Approved! Abbott FreeStyle Libre® 3 Continuous Glucose Monitor, Ages 4+
The FDA cleared the FreeStyle Libre 3 continuous glucose monitor (CGM) for children and adults ages 4 and up with diabetes

Smart Insulin Pen/Smart Cap Bridge Technology Gap for MDI
People living with T1D who prefer multiple daily injections (MDI) of insulin now have two high-tech management options: An insulin smart pen or an insulin smart pen cap.

Abbott FreeStyle Libre 2 iOS App Receives FDA Approval
On August 2, 2021, Abbott announced the FreeStyle Libre 2 iOS app received approval from the FDA for adults and children ages 4 and up with diabetes.

Bigfoot Is Real—and Received FDA Clearance
The Bigfoot Unity™ Diabetes Management by Bigfoot Biomedical received FDA clearance for individuals 12 and older.

Tandem Diabetes Care in the Real World
Two leaders from Tandem Diabetes Care share real-world outcomes from Tandem insulin pump users, other practical insights.

Breakthrough T1D and Tandem Diabetes Care Partner to Help Educate, Support the T1D Community
New, national partnership will use Breakthrough T1D’s community-facing platforms to highlight benefits of devices such as insulin pumps, continuous glucose monitors and novel treatment approaches.

Nation’s Largest Insurer Expands Insulin Pump Coverage
In a victory for Breakthrough T1D’s Coverage2Control Campaign, people with T1D now have more choice in how they manage their diabetes.

The Importance of Dexcom CLARITY: From Telemedicine to Ultra Marathons
Elite T1D Athlete Eric Tozer and Dexcom Certified Diabetes Educator (CDE) Cher Pastore discuss Dexcom CLARITY technology.