
Breakthrough T1D Advocates Unite in D.C. to Fight for the T1D Community
The Federal Government is one of Breakthrough T1D’s most important partners in advancing research to better treat, prevent, and ultimately cure type 1 diabetes (T1D). Breakthrough T1D’s annual Government Day event, held in Washington, D.C., brings together dedicated Breakthrough T1D Advocates from around the nation to use their own personal stories to communicate the financial, […]

Breakthrough T1D Presents Award Honoring Trailblazer Mary Tyler Moore
The inaugural award goes to Senator Susan Collins (R-ME), Senator Jeanne Shaheen (D-NH), and Rep. Diana DeGette (D-CO).

Meet Natalie Stanback: Breakthrough T1D 2023 Children’s Congress Chair
She knows T1D; she knows Advocacy—and she knows how to get things done on Capitol Hill! Meet the Breakthrough T1D 2023 Children's Congress Natalie Stanback.

National Clinical Care Commission Final Report Published
The National Clinical Care Commission issued its report to Congress, which included a recommendation for a five-year renewal of the Special Diabetes Program.

Special Diabetes Program (SDP) Renewed for Three Years!
One of Breakthrough T1D’s top legislative priorities has been achieved with the enactment of a three-year renewal of the Special Diabetes Program (SDP). The program, now funded until September 30, 2023 at $150 million per year, will enable researchers to build upon the momentum in type 1 diabetes (T1D) to date and explore new opportunities […]

Cass Freeland Builds Relationships with Political Power Players
NDAM Profile: Breakthrough T1D advocate Cass Freeland’s expertise in connecting with policy makers demonstrates The Power of Us.

FDA Approves an Artificial Pancreas System for Children Aged 2-6 Years
The Food and Drug Administration (FDA) approved the Medtronic MiniMed 770G artificial pancreas system for use by children aged 2 to 6 with type 1 diabetes (T1D). It is the first marketed device that can automatically adjust insulin delivery based on continuous glucose monitoring (CGM) values for children aged 2-6 years.
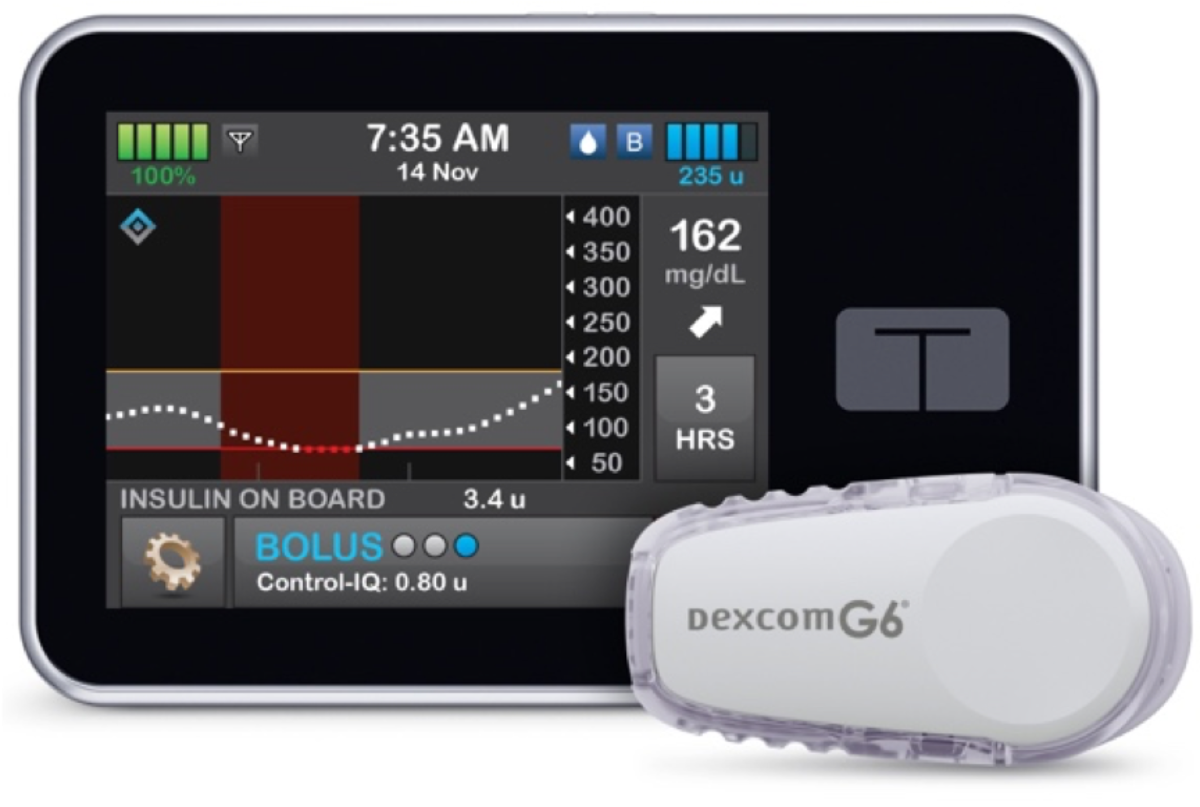
Tandem Control-IQ—Now Authorized For Children
Tandem Diabetes announced FDA clearance of an expanded pediatric indication for the Tandem t:slim X2™ insulin pump with Control-IQ™ technology in children ages six and older.

Enhanced Charitable Giving Incentives Explained: What it Means for Donors in 2020
What the federal economic relief package, also known as the CARES Act (Coronavirus Aid, Relief, and Economic Security) means for donors to Breakthrough T1D.

Special Diabetes Program Funding Extended Allowing Critical Type 1 Diabetes Research to Continue
The Federal Government has continued its commitment to support diabetes research by providing more than $75 million into the Special Diabetes Program (SDP), which drives forward critical research aimed at curing type 1 diabetes (T1D) and improving the lives of those living with the disease today. The funding, approved by both the House and Senate and signed by the […]