
Tandem Diabetes Care in the Real World
Two leaders from Tandem Diabetes Care share real-world outcomes from Tandem insulin pump users, other practical insights.

Just Doing Experiments? Well, Ones that May Have Life-Saving Potential
Smart insulin; what is that? It’s the type of insulin that Chandra Bhattacharya, Ph.D., has spent her Breakthrough T1D-funded postdoctoral fellowship—the time right after you receive your doctoral degree, but are not ready for primetime as an assistant professor, yet—to pursue. And she has a lot of shoes to fill. At the Massachusetts Institute of […]

Breakthrough T1D and Tandem Diabetes Care Partner to Help Educate, Support the T1D Community
New, national partnership will use Breakthrough T1D’s community-facing platforms to highlight benefits of devices such as insulin pumps, continuous glucose monitors and novel treatment approaches.

Nation’s Largest Insurer Expands Insulin Pump Coverage
In a victory for Breakthrough T1D’s Coverage2Control Campaign, people with T1D now have more choice in how they manage their diabetes.

The Importance of Dexcom CLARITY: From Telemedicine to Ultra Marathons
Elite T1D Athlete Eric Tozer and Dexcom Certified Diabetes Educator (CDE) Cher Pastore discuss Dexcom CLARITY technology.

Severe hypoglycemia: Be prepared, not afraid
Fear of Hypoglycemia is a real thing. Learn how you and your loved ones with T1D can overcome it.

FreeStyle Libre 2 Authorized for Use in the U.S.
On June 15, 2020, Abbott announced that the FDA has authorized the FreeStyle Libre 2 System. The Libre 2 gained its CE Mark clearance in Europe late 2018 and is now authorized for adults and children 4 and up in the United States. The FreeStyle Libre 2 is a 14 day continuous glucose monitor (CGM) […]

Snails—Yes, Snails—Are Helping Unlock Faster Insulins
Scientists have uncovered a potential breakthrough in developing a faster acting insulin in an unusual place: snails. Now, Breakthrough T1D-funded researchers are using the molecular features of the snail insulin to create ultra-rapid insulins for people with type 1 diabetes (T1D) and have published their findings in . As discussed in last year’s blog, certain […]

Medtronic Panel Discusses Supporting T1D Community During COVID-19
During a recent Breakthrough T1D Facebook Live event, a panel of leaders discussed Medtronic’s efforts to support the T1D community during the pandemic.
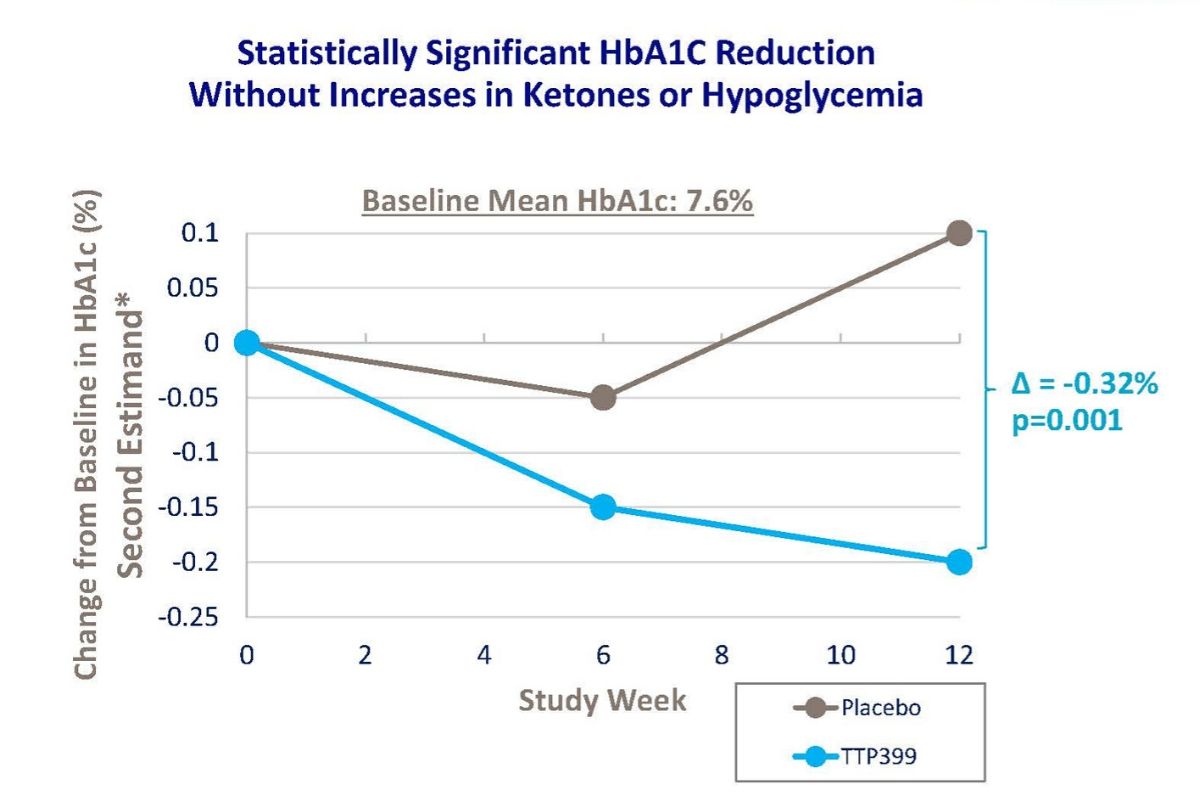
TTP399: A Potential First-in-Class Adjunct Therapy in Type 1 Diabetes
Nearly 80 percent of people with type 1 diabetes (T1D) fail to meet HbA1c goals defined by the American Diabetes Association. Despite more widely adopted diabetes technology and an increase in use, there is no improvement in clinical outcomes. This tells us that the current use of insulin alone is not enough, but vTv Therapeutics […]