
European Commission Has Approved Forxiga for Adults with Type 1 Diabetes
It’s official: The European Commission has approved Forxiga® (dapagliflozin and marketed in the United States as Farxiga®) for adults with type 1 diabetes (T1D), when used in conjunction with insulin to improve glycemic control. This is the first oral medicine for T1D in Europe, and blocks SGLT, which is responsible for glucose absorption in the […]

Mini, AP Patch Given FDA Nod for Fast-Track Review
A closed-loop patch insulin pump/CGM system has been granted FDA Breakthrough Device Designation by the FDA. The designation is designed to provide a faster track to FDA approval for devices that are considered to provide novel treatment options. The device was created by EOFlow, a company based in South Korea, and the technology was funded […]

Eric Tozer World Marathon Challenge Recap
7 Marathons? Check. 7 Continents? Check 7 Consecutive Days? Check We checked in with Eric Tozer to ask him about his experience running the World Marathon Challenge, which he became one of the first people with type 1 diabetes to complete a few weeks ago. How does it feel to have completed the World Marathon […]

The 20th Anniversary of the Edmonton Protocol
This day, 20 years ago, a person with type 1 diabetes (T1D) was cured. Well, pseudo-cured. On March 11, 1999, the Edmonton Protocol had its first participant. This ground-breaking clinical trial was testing a new type of islet transplantation, without corticosteroid immunosuppressive drugs (which is necessary for the prevention of graft rejection). The seven people […]
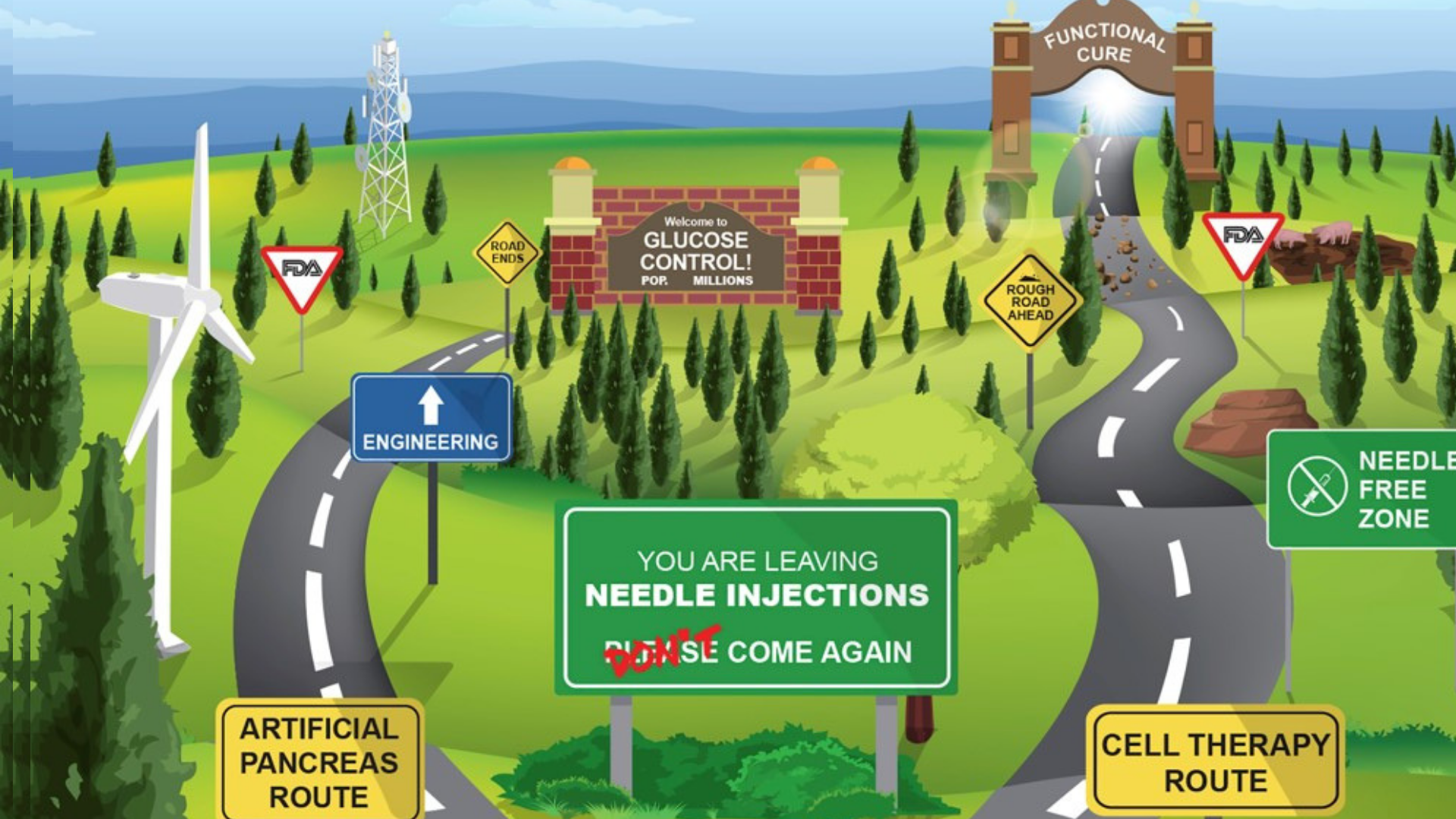
Insulin Discovered Nearly 100 Years Ago: How Far We’ve Come and the Road Ahead
Incredible strides have been made since the discovery of insulin almost 100 years ago. Insulin formulations have improved dramatically, blood-sugar levels can be measured continuously and first-generation artificial pancreas systems have reached the market. Yet only a small percentage of people with type 1 diabetes (T1D) achieve their blood sugar goals. In a review published […]

Advocacy in Action: Breakthrough T1D 2019 Government Day
After nearly 500 meetings with Members of Congress, Breakthrough T1D 2019 Government Day has officially come to a close. Over the course of four days, 175 grassroots leaders from across the country came to Washington, D.C. to advocate for the top issues affecting the type 1 diabetes (T1D) community. These issues, outlined in the Breakthrough […]

JDRF-Backed Start-Up Company to Accelerate Work Through Pfizer
AnTolRx, Inc., a private biotechnology company, has agreed to license its immune tolerance therapy for type 1 diabetes (T1D) to Pfizer with the aim of advancing the research and accelerating its potential for commercialization. The research is part of a larger portfolio of work done by AnTolRx, a Cambridge, Massachusetts-based research and development biotechnology company. […]

Eli Lilly Announces Lower-Cost Generic Insulin
Eli Lilly and Company announced it will begin selling a generic version of its most commonly prescribed insulin -- Humalog

Can a Snail Hold the Key to Better Blood Sugar Control?
Helena Safavi, Ph.D., of The University of Utah, decided to take an unusual and creative approach to ultra-rapid insulin development, studying sea snails that use fast-acting insulin, not as a hormone to regulate their blood glucose, but as a toxin to sedate their prey.

Tracking Oxygen Levels Around Transplanted Cells Could Help Build Successful Beta Cell Replacement Therapies
Understanding change in oxygen levels over time in mice may help researchers develop better methods of replacing beta cells in humans.