100, 100: Genetically Engineered Human Insulin Created
In 1976, researchers set out to synthesize the human insulin gene and make human insulin, and Breakthrough T1D researchers had multiple hands in the biosynthesis of it.

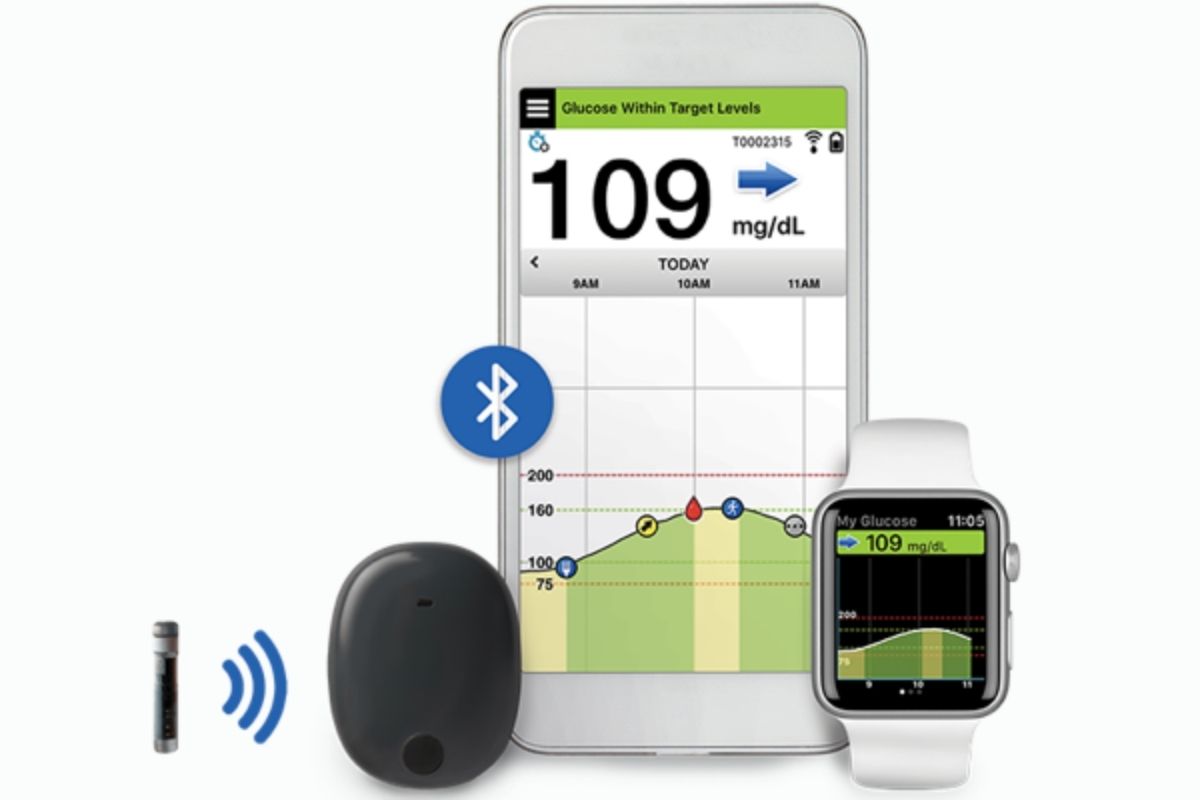
More CGMs Now Covered by Medicare
In March 2022, Medicare beneficiaries became eligible for coverage for a greater number of CGMs than ever before. Due to a final rule issued on December 21, 2021 by the Centers for Medicare and Medicaid Services (CMS), any CGM that connects with an insulin pump or a standalone receiver will be covered by Medicare for […]

Insulin Affordability? Yes, Please: Breakthrough T1D Joins Civica to Make Low-Cost Insulin Available to All Americans
In conjunction with leading partners, Breakthrough T1D lends its support to Civica, to help combat a nationwide insulin affordability crisis.

Breakthrough T1D Statement: President’s Comments about Insulin Affordability During State of the Union
Breakthrough T1D is glad President Biden used the State of the Union to address the insulin affordability crisis, which has had devastating consequences in the diabetes community for far too long. Joshua Davis, the young man with type 1 diabetes who joined the First Lady in the gallery, was one of the Breakthrough T1D Children’s […]

Helping the Diabetes Community in Ukraine – 6/1 Update
Guide on how to support the diabetes community in Ukraine.

Breakthrough T1D is in Animal Crossing™ New Horizons!
When Insulet, the makers of Omnipod®, asked us to team up on bringing diabetes representation to Animal Crossing™, we couldn’t say no!

Tandem Diabetes Makes Managing T1D More Convenient
Tandem announced the FDA clearance of bolus insulin dosing from their t:connect® mobile app. Soon, individuals with the Tandem t:slim X2™ pump will be able to bolus remotely from either their iOS or Android smartphone.

A Mother on a Mission to Cure T1D
“What drives us as parents is the love we have for our children and the passionate hope that there is a cure out there and it’s within reach.” – Lee Ducat
FDA Approval of Senseonics Eversense® CGM for Use for Up to 6 Months
FDA approves Senseonics Eversense® E3, the first long-term implantable CGM system, and the E3 includes technology that extends the use for up to 6 months.

100 Years, 100 Breakthrough T1D Scientists: The BB Rat—An Animal Model of Type 1 Diabetes
In 1974, a colony of non-diabetic BB rats develops diabetes. Examination of their pancreases revealed that they had no beta cells.