What’s happening?
Continuous ketone monitoring (CKM) is on its way. A new paper in The Lancet Diabetes & Endocrinology, titled “International expert recommendations on the application and utility of continuous ketone monitoring for people with diabetes,” was published by Breakthrough T1D and expert collaborators, spearheaded by our Chief Medical Officer, International, Thomas Danne, M.D., Ph.D. This publication summarizes the recommendations for effective use CKM use in people with type 1 diabetes (T1D) at risk of developing diabetic ketoacidosis. Read on to learn more about this technology, guidelines for its use, and what this means for the future.
Ketones and DKA: What you need to know
When there is not enough insulin in the body, glucose cannot be used for energy, and the body uses fat for fuel instead. Excessive breakdown of fat ultimately causes ketones to build up in the blood. Very high levels of ketones in the blood can lead to diabetic ketoacidosis (DKA)—a life-threatening condition that requires immediate medical treatment.
Too often, people are diagnosed with T1D only after they have experienced DKA. Yet, even for people who are aware that they have T1D, DKA can and does happen. There are many reasons that it can occur, including the onset of illnesses, like the flu. Currently, people with diabetes use blood and urine strips to test for ketones—but even so, surveys show that very few people measure their ketones regularly, and DKA rates remain unacceptably high.
Given the current options, it remains very difficult to predict when ketone levels may be rising, and people lose opportunities to intervene early to prevent DKA altogether. So, how can we fill this gap?
The answer: continuous ketone monitoring.
CKMs are coming—but they’re not here yet
CKMs are in the works, and we can expect them to become a reality sooner rather than later. Similar to continuous glucose monitors (CGMs), CKMs will continuously measure ketone levels in the body and can alert users if their ketone levels are rising. This ability to track ketone levels in real-time could have substantial benefits in preventing DKA—and more.
Examples of people with T1D who may benefit from CKM
- Insulin pump users, including automated insulin delivery (AID) users, who are at higher risk for DKA due to possible infusion-set or infusion-site failures
- People with T1D and complications, such as chronic kidney disease or cardiovascular disease, who have an increased risk of DKA
- Pregnant women with pregestational or gestational diabetes, who may have increased resistance to insulin and therefore higher rates of DKA
As of today, there are academic groups and early-stage companies working on CKM. Abbott also has a glucose-ketone sensor (CGM-CKM) in development. Other companies, including Tandem, Beta Bionics, Sequel Med Tech, and Ypsomed, are planning to integrate Abbott’s ketone-glucose sensing device into their AID systems once it is available. We’re also keeping an eye a company called SIBIONICS, which currently offers a wellness-focused CKM that has demonstrated strong ketone-tracking data, although it is not available as a medical device yet.
Breakthrough T1D strongly supports the development of CKM to help protect people with T1D from the dangers of DKA. We previously funded five projects—two of which are co-funded by the Helmsley Charitable Trust—to support the development of CGM-CKM. Now, we’re getting ahead of the curve for CKM so we can learn how to use this technology to answer clinical questions about T1D.
We are currently looking for and investing in research projects to understand how ketone monitoring may improve outcomes for people living with T1D from multiple angles, including DKA prevention, understanding disease progression and pathophysiology, learning from clinically meaningful ketone patterns, and more. The goal is to accelerate the generation of evidence surrounding the use of CKM while these devices are on the horizon. This way, we can get a better idea of the kinds of benefits this technology can bring to the T1D community.
Snapshot of CKM recommendations for people with T1D
We feel optimistic that CKMs will become a reality soon. The next question is: how should they be integrated into T1D care? To answer this question, Dr. Danne and an international Breakthrough T1D consensus group published a report about how these novel technologies can be effectively used to help the T1D community.
The experts agreed on the following recommendations:
- CKMs should include trend arrows, akin to those on a CGM, and reflect rates of change of around 0.4 mmol/L per hour.
- Due to the existence of alarm fatigue, alarms should sound if ketone concentrations rise above the threshold of ≥3.0 mmol/L.
- The terminology for ketone levels should be: Normal, Elevated, High, Urgent High.
- All CKM users should be provided with a blood ketone meter to use if they are experiencing the symptoms of High or Urgent High ketones that do not match the CKM reading.
- All individuals who wear a CKM should receive education on what elevated ketones mean and what actions to take.
These are just a few of the highlights. Check out the full publication for a more in-depth look at what the experts recommend.
Why CKM guidelines are important
CKMs have the potential to transform T1D care by stopping dangerous, life-threatening DKA before it ever occurs. It is critically important that healthcare professionals (HCPs) and people living with T1D know how to most effectively use this technology to maximize its benefits.
This publication does just that. It’s a set of established guidelines, put together by experts from around the globe, to help standardize the integration of CKM into clinics once they become available. Both HCPs and the T1D community can refer to these recommendations as this technology is rolled out—and potentially becomes the standard of care—to start realizing the benefits of CKM as soon as possible.
Even more, this document can be used as a resource in future conversations surrounding regulatory decisions and payer coverage. Ultimately, we are preparing all stakeholders for a future that we know will be here soon—one where CKM is a valuable addition to daily T1D management.

“Experts from 12 countries agree: continuous ketone monitoring can be a game-changer. By detecting risk earlier and guiding faster action, CKM has the potential to reduce DKA, strengthen confidence for people with T1D, and transform how we deliver safe, proactive diabetes care.” – Thomas Danne, M.D., Ph.D., Chief Medical Officer, International
How this fits into our Medical Affairs goals
CKMs are coming—how can we ensure they make their way into clinics around the globe? By preparing the T1D community in advance of their arrival, we are setting the stage for CKMs to come. That way, once they are available, HCPs and people with T1D will already know what to do.
In essence, this is clinical adoption: the cornerstone of our Medical Affairs unit. We are preparing the right people at the right time by providing resources, education, and guidelines for a new technology. We are getting our ducks in a row before the new technology is here—so that we are 100% ready when it is.
Thanks to Dr. Danne and the expert consensus panel, we can be ready-as-ever for CKMs to materialize. We look forward to a future in which DKA is easily prevented and the T1D community can fully experience the benefits of continuous ketone monitoring.
How quickly a year goes by—and a lot has happened for type 1 diabetes (T1D) in 2025! In these last few weeks leading up to the new year, Breakthrough T1D would like to take a moment to reflect on how far we’ve come in the past 365 days. Read on for the greatest T1D accomplishments in 2025—and the exciting things we’re looking forward to in 2026.
A special note for our donors
Breakthrough T1D would like to give a special thank you to our donors. Each and every one of us —staff, volunteers, T1D community members, researchers, advocates, clinicians, and more—are able to accomplish what we can because of you. Our shared commitment to a world without T1D is driven by your generosity, and truly no words can adequately express our appreciation for your continued support of our mission. Thank you for all that you do!
Check out our Top Advances 2025 video featuring Breakthrough T1D CEO Aaron Kowalski, Ph.D., and read on for more details!
Cell therapy milestones
First things first: cell therapies. One of the biggest breakthroughs that had the T1D community buzzing in the last year was Sana Biotechnology’s donor-derived islets that are genetically engineered to be invisible to the immune system. These cells are still making insulin 6+ months after the first person was treated—without immunosuppression! Sana—a portfolio company of the T1D Fund: A Breakthrough T1D Venture—published this accomplishment in The New England Journal of Medicine.
On the manufactured cell therapies front, Vertex Pharmaceuticals continues to hit milestones with zimislecel (formerly VX-880)—a therapy that Breakthrough T1D has had a hand in for decades. Promising results from the phase 1/2/3 clinical trial were published in The New England Journal of Medicine, revealing that 10 of 12 participants (83%) are insulin therapy independent. The study is ongoing.
Tegoprubart, an immunomodulatory drug made by the T1D Fund portfolio company Eledon, is being investigated as an alternative to standard immunosuppressants to help prevent transplanted islets from rejection. In a Breakthrough T1D-funded trial, six individuals who have received a donor islet transplant and tegoprubart are now insulin therapy independent. Preliminary data suggest that tegoprubart is more tolerable and has milder side effects compared to standard immunosuppressants—paving the way for easier post-transplant management. The study is ongoing, and nine people total are enrolled. The promising results from this trial opened the doors to another Breakthrough T1D-funded study that will include people with T1D and chronic kidney disease (CKD)—a population that was previously excluded from islet transplants due to side effects associated with standard immunosuppressants.
Cell Pouch is an implantable device that provides a livable environment for transplanted islets to help them survive. The ongoing phase 1/2 trial investigating Cell Pouch with donor-derived islets is on track to meet its endpoints for safety and tolerability, and people are achieving insulin therapy independence. The final cohort of this trial, which is expected to initiate before the end of this year, will use manufactured islets provided by Sernova’s partner Evotec. Even more, this cohort will receive Eledon’s tegoprubart instead of standard immunosuppressants—accelerating progress on two fronts at once. Breakthrough T1D has been supporting the development of Cell Pouch over many years, and we look forward to seeing data from the final cohort.
Vertex and large-scale manufacturing
While zimislecel is in the works, Vertex has forged collaborations to manufacture these cells at a large scale once they are approved. They’ve partnered with Lonza to build a dedicated large-scale facility for eventual commercial production of zimislecel. They’ve also licensed technology from TreeFrog to scale up production capabilities. We call that planning ahead!
Civica’s low-cost, long-acting insulin available on January 1, 2026
Thanks to a huge win for insulin affordability, the first day of 2026 will kick off with a bang. Civica Rx’s insulin glargine-ygfn (which is interchangeable with Lantus®), will be available on January 1, 2026, for no more than $55 for five pens. Anyone with a prescription—regardless of insurance status—will be able to purchase it at a pharmacy. Breakthrough T1D has been working with Civica for nearly three years to make this possible.
Positive outlook for disease-modifying therapies
Building on results from a promising study funded by Breakthrough T1D, Eli Lilly launched two new pivotal clinical trials for baricitinib, a JAK inhibitor that is already approved for other autoimmune diseases. The trials will test whether baricitinib—which is taken as a once-daily oral pill—can delay the progression of stage 2 to stage 3 T1D (BARICADE-DELAY) or preserve beta cells in newly diagnosed stage 3 T1D (BARICADE-PRESERVE). Enrollment will open in 2026.
Another disease-modifying therapy is coming down the pike: ATG. Following from prior studies, the phase 2 MELD-ATG clinical trial found that low-dose ATG has the potential to preserve insulin-producing beta cells in children and young adults between five and 25 years old, and it was generally well-tolerated. In line with ATG’s potential, SAB Biotherapeutics—a company with funding support from the T1D Fund—is developing a next-generation ATG therapy.
Tzield, the first disease-modifying therapy approved to delay stage 3 T1D in people eight years and older in stage 2 T1D (before insulin therapy is required), has been accepted into the FDA Commissioner’s National Priority Voucher (CNPV) program for accelerated review. If approved, this could lead to expanded use of Tzield for people with newly diagnosed stage 3 T1D (when insulin therapy is required)—and this would be the first time people at this stage could have a therapy option besides insulin. We look forward to hearing more soon.
Right now, Tzield is only approved for use in delaying progression from stage 2 to stage 3 T1D for kids and adults eight years and older. The PETITE-T1D clinical trial is trying to change that. Interim results showed that Tzield is safe and well-tolerated in children under eight with stage 2 T1D, with no new safety issues reported. The study is ongoing and final results are expected in 2026.
Tzield in action: Breakthrough T1D’s Chris Dunn
Chris Dunn is a parent of four children, two of whom live with T1D. Knowing that immediate family connections are a risk factor for developing T1D, Chris and her children without T1D participated in screening. Much to her surprise, Chris learned that she was in stage 2. Chris knew that Tzield could potentially delay her need for insulin therapy, so she made the informed decision to try it.
A big win for kidney disease, plus new trials, devices, and more to improve the lives of people with T1D
Bayer shared data from the phase 3 FINE-ONE clinical trial, which investigated finerenone (Kerendia®)—a drug that is already approved for type 2 diabetes—for CKD associated with T1D. The results showed that finerenone significantly reduces a marker of kidney damage, marking the first treatment in nearly 30 years to achieve positive outcomes for CKD in people with T1D. This data will be submitted for regulatory review to expand finerenone’s indication to include T1D.
The Breakthrough T1D-funded phase 2 ADJUST-T1D trial investigated whether the GLP-1 receptor agonist semaglutide (Ozempic®) could benefit people living with T1D and obesity or overweight who are using an automated insulin delivery (AID) system. Thirty-six percent of people in the trial treated with semaglutide met the primary endpoints for glycemic and weight control compared to 0% in the placebo group, and the drug was well-tolerated and safe. These data—which were published in The New England Journal of Medicine Evidence—show that GLP-1 receptor agonists have the potential to help people with T1D manage both blood sugar and weight.
Building on the growing interest in GLP-1s for T1D, Eli Lilly launched two pivotal clinical trials for tirzepatide (Mounjaro® or Zepbound®), a dual GLP-1/GIP receptor agonist. These trials—which are enrolling now—will investigate whether tirzepatide can reduce blood sugar levels in people living with T1D and obesity or overweight over a period of 40 weeks (SURPASS-T1D-1) or 20 months (SURPASS-T1D-2). Industry investment in GLP-1s for T1D is a promising avenue toward a future where this class of drugs may be approved for use in members of the T1D community.
AID systems are getting better each year, and approximately 10 such systems are now approved for use in the United States. Here are a few advances in diabetes technology that stood out:
- Dexcom’s G7 15-day sensor—the longest-lasting FDA-approved wearable continuous glucose monitor (CGM) on the market—is now available for people in the U.S. 18 and over.
- Sequel Med Tech’s twiist AID system, which uses FDA-cleared Tidepool Loop technology, is now available in the U.S.
- Tandem’s SteadiSet 7-day infusion set has been cleared by the FDA and is expected to launch in the U.S. in 2026.
- Abbott’s Freestyle Libre 3 Plus sensor has integrated with twiist and T:slim X2 insulin pumps.
- Medtronic unveiled the largest real-world dataset pulled from users of the MiniMed 780G AID system.
To better understand the mental health needs of the T1D community, Breakthrough T1D and the Helmsley Charitable Trust co-hosted a Psychosocial Roundtable, convening mental and behavioral health experts across the T1D care spectrum. The discussion revolved around challenges and gaps in psychosocial care for people with T1D and how we can work together to address them. Thanks to the expert opinions of the attendees, Breakthrough T1D has a clear path forward for tackling these issues and supporting the mental and emotional wellbeing of the T1D community through research and awareness.
Making headway in rebalancing the immune system in T1D
Sanofi has entered into a licensing agreement with EVOQ Therapeutics, a company with support from Breakthrough T1D, to help develop and commercialize its NanoDisc technology. This technology has the potential to retrain the immune system to stop attacking beta cells. Industry partnerships like these are key to accelerating T1D research.
The Nobel Prize in Physiology or Medicine was awarded to three immunologists—Fred Ramsdell, Ph.D., Mary E. Brunkow, Ph.D., and Shimon Sakaguchim M.D., Ph.D.—for their discoveries in immune tolerance, meaning the immune system’s ability to distinguish between self and non-self. Building on these breakthroughs, Dr. Ramsdell co-founded Sonoma Biotherapeutics, a T1D Fund-backed company, to restore immune tolerance in autoimmune diseases like T1D. Another year, another researcher with ties to Breakthrough T1D winning the most prestigious award in science!
Launch of Breakthrough T1D’s Medical Affairs department
A noteworthy organizational change is marked by the launch of Breakthrough T1D’s Medical Affairs unit, which aims to bridge the gap between access to and adoption of T1D therapies, devices, and treatments. The team—which continues to expand—is tackling challenges associated with slow clinical adoption of T1D therapies. In less than a year, the team has already launched healthcare professional (HCP) education and resources and hosted two international workshops at Breakthrough T1D HQ focused on preparing the clinical workforce to bring next-generation cell replacement therapies to the T1D community. This is just the beginning, and we can’t wait to see what else our Medical Affairs unit will accomplish!
Continuous ketone monitoring consensus guidelines published
Continuous ketone monitoring (CKM) has substantial benefits for the T1D community, including preventing diabetic ketoacidosis. CKMs are coming—and to prepare, Breakthrough T1D’s Chief Medical Officer, International Thomas Danne, M.D., Ph.D., spearheaded an effort with expert collaborators to publish consensus guidelines for use of CKM in T1D. These guidelines will help HCPs integrate CKM into clinics and establish best practices for their use in the T1D community.
Tackling T1D on a global scale
Breakthrough T1D, in partnership with the Helmsley Charitable Trust and Roche and Sanofi’s Global Health Unit, launched ALIGN-T1D. This is a new global alliance uniting philanthropic, industry, and community leaders to strengthen government-led T1D care networks in low-middle income countries, integrating diagnosis, insulin access, monitoring, and education.
Breakthrough T1D helped organize an event at the European Parliament to highlight unmet needs for the T1D community and discuss how to accelerate cures—especially cell therapies—in the European Union. In a similar vein, an event co-hosted by Breakthrough T1D in Brussels, Belgium sparked conversation about overcoming challenges surrounding cardiovascular disease in T1D. Building long-term partnerships with EU Institutions and international diabetes organizations will allow us to work together towards global T1D breakthroughs.
Breakthrough T1D, in partnership with Friends of Mewar and UNICEF, participated in the Udaipur Type 1 Diabetes Summit: Advancing Access, Equity, and Action, in Udaipur, Rajasthan, India. The event gathered government leaders, health experts, community advocates, and international partners to develop a collaborative roadmap for strengthening T1D care across India.
Breakthrough T1D, Breakthrough T1D Canada, and the Stem Cell Network partnered to support four new projects led by Canadian researchers to drive high-impact research into manufactured cells and next-generation cell replacement therapies. This partnership is a novel and meaningful part of Breakthrough T1D’s global Project ACT effort to power high-impact cell therapies research.
The Australian government committed $50M over five years to the T1D Clinical Research Network (T1DCRN), a highly impactful research network driven by Breakthrough T1D Australia. This funding will support T1D research in prevention, precision medicine, and cures.
The Grand Challenge, a partnership between Breakthrough T1D UK, the Steve Morgan Foundation, and Diabetes UK, is funding 23 research projects to advance new treatments and cures for T1D. Their funding is supporting 189 researchers and collaborators across 49 institutions in eight countries. Recent breakthroughs from the past year include uncovering why T1D could be more aggressive in young children and developing a new insulin-glucagon molecule that could reduce dangerous hypoglycemic events.
Breakthrough T1D publications across the map
Breakthrough T1D Research and Advocacy staff, in collaboration with other leading experts in the field, authored a paper titled “Future Directions and Clinical Trial Considerations for Novel Islet Beta Cell Replacement Therapies for Type 1 Diabetes” in the journal Diabetes. This publication outlines what the future of beta cell replacement therapies looks like—and how we can make these therapies a reality for everyone with T1D who wants them through innovative clinical trial design and expanding the pool of eligible trial participants.
“The Urgent Need for Breakthrough Therapies and a World Without Type 1 Diabetes” was authored by Breakthrough T1D staff and leadership and published in Diabetes Therapy. This commentary exposes the many persistent challenges of living with T1D, despite incredible progress in recent years. The publication is a call to action for the entire T1D community—researchers, clinicians, advocates, people with T1D, regulators, and all other stakeholders—to work together to overcome barriers at all levels to make cures for T1D a reality sooner rather than later.
Breakthrough T1D Advocacy staff and collaborators published a paper titled “Perceptions of the Benefits and Risks of Novel Therapies for Type 1 Diabetes: A Qualitative Study” in the journal Diabetes Therapy. This Breakthrough T1D-funded study found that people with T1D and their caregivers are largely willing to try next-generation therapies, drawn by the promise of reduced reliance on insulin and freedom from constant disease management. These findings highlight the importance of ensuring that the perspectives of people with T1D and their caregivers are used to guide regulatory decisions around the benefits and risks of a new therapy.
Breakthrough T1D Advocacy staff and collaborators published an article called “We Are on the Verge of Breakthrough Cures for Type 1 Diabetes, but Who Are the 2 Million Americans Who Have It?” in The Journal of Health Economics and Outcomes Research. This study identifies key T1D demographics in the U.S. and predicts how these demographics may change over the next decade. These data will help inform elected officials and payers about how healthcare policies may ultimately affect the T1D community—ensuring that the policies in place are having the greatest impact they can.
An analysis by Avalere Health, which was supported by Breakthrough T1D, found that research funded by the Special Diabetes Program (SDP) has yielded more than $50 billion in federal healthcare savings. This study supports the fact that the SDP has a strong return on investment, both clinically and economically, and demonstrates its ever-important role in bringing advanced therapies to the T1D community and improving health outcomes. Even more, SDP funding was recently extended through January 30, 2026, with retroactive funding back to October 1, 2025.
Consensus guidance on population screening for T1D coming soon
Breakthrough T1D’s Vice President of Medical Affairs, Anastasia Albanese-O’Neill, Ph.D., APRN, CDCES, spearheaded an effort to establish a consensus on T1D screening guidance. These guidelines—which will be published soon—push for population-level T1D screening and provide guidance for HCPs to effectively integrate T1D screening into their clinics. The publication will outline the benefits, harms, and methods of T1D screening, who should be screened and how often, and how to effectively communicate screening results.
Breakthrough T1D announces first Clinical and Research Congress in October 2026
In a historic first, Breakthrough T1D is hosting our very own Clinical and Research Congress next fall! This meeting will bring together clinicians, researchers, scientists, HCPs, industry leaders, and the T1D community to explore cutting-edge advances in T1D research, clinical care, health equity, and more. The goal is to foster collaboration and spark innovation to drive our mission forward faster than ever.
Bonus advance: The first Barbie doll with T1D
In December 2023, Mattel approached Breakthrough T1D with a partnership opportunity for the first Barbie with T1D. Less than two years later, in July 2025, Barbie with type 1 diabetes was launched to a most enthusiastic reception from Delegates at Breakthrough T1D’s Children’s Congress. This groundbreaking global collaboration reflects a shared commitment to ensuring that the millions of people living with T1D are seen, heard, and empowered. It’s working—the dolls have been in high demand since launch!
One word: WOW! That’s a lot of exciting things that YOU helped make possible. Thank you for your support of Breakthrough T1D and everything you do to drive our mission forward. Each year, each month, each day, we get closer to a world without T1D. This wouldn’t be possible without our donors, community members, staff, leadership, and every single heart and mind around the globe affected by the work we do each day. Cheers to a successful 2025—and here’s to another year of accomplishments!
What’s happening?
Today, Breakthrough T1D co-hosted an event with leading international diabetes organizations to discuss cardiovascular disease (CVD) and type 1 diabetes (T1D)—where we are, challenges that remain, and how we can work together in Europe and beyond to address this need for the T1D community.
The focus of the event: Doing more for CVD and T1D

This event, titled “Type 1 Diabetes & Cardiovascular Disease: From Data to Solutions,” took place today on November 14, 2025, in Brussels, Belgium. Breakthrough T1D co-hosted this event in conjunction with the International Diabetes Federation (IDF) Europe and the International Society for Pediatric and Adolescent Diabetes (ISPAD). The goal was to bring together different stakeholders to address a critical concern: the lack of therapies for cardiovascular disease for people living with T1D.
The speakers at the event covered a range of topics. Jonathan Rosen, Ph.D., Director of Research at Breakthrough T1D spoke about the often-overlooked link between CVD and T1D. Others touched upon lessons we can learn from (T2D) diabetes and CVD, the gaps in evidence that still exist, new frontiers in CVD care for people living with autoimmune diseases, and the inclusion of T1D in CVD therapy development.
Attendees
From Breakthrough T1D:
- Sanjoy Dutta, Ph.D., Chief Scientific Officer
- Jonathan Rosen, Ph.D., Director of Research
- Carmen Hurtado del Pozo, Director of European Research
- Hilda Ahnstedt, Program Officer of European Research
- Alessandro Bisio, M.D., Director of Medical Affairs, International
Other attendees:
- Speakers from the European Medicines Agency
- Member of the European Parliament: Elena Nevado del Campo (EPP, Spain)
- People living with T1D
- Researchers
- Industry leaders
Why this matters

People living with T1D have a high risk of developing heart complications, despite advances in T1D care. At this time, there are only a few options available to lower this risk. In the United States and Europe, people with T1D have access to blood pressure medications and lipid-lowering medications (for example, statins) that can reduce the risk of developing CVD.
Other therapies, like the SLGT inhibitors empagliflozin or dapagliflozin, are the standard of care in the U.S. to treat heart failure in people without diabetes or with T2D. This class of drugs has revolutionized treatment and significantly reduced the rates of heart failure in these populations. However, people with T1D were excluded from these clinical trials—despite the fact that heart disease remains a critical concern for the T1D community. While not strictly approved, these drugs are occasionally prescribed off-label to reduce the risk of heart failure in people with T1D. Greater accessibility will require regulatory approval, and these options are not currently available for people living in Europe.
Despite the availability of some treatments that can reduce the risk of heart complications, there are no available cardioprotective therapies in the U.S. or Europe that can prevent CVD in people with T1D. More targeted, innovative therapies and treatment strategies are needed to further reduce the risk of—or ideally prevent altogether—cardiovascular complications in the T1D population.
There is a clear unmet need for the T1D community: the risk of CVD is high, and there are not enough options to treat or prevent it. That’s where events like these come in. They are a call to action for collaborative research efforts, better data integration, new and innovative ideas, and global cooperation towards a shared goal of better heart health for people living with T1D. The growing prioritization of CVD is evidenced by the European Commission’s cardiovascular health plan—which includes the Joint Action on Cardiovascular Diseases and Diabetes—showcasing how stakeholders in Europe are taking charge to accelerate breakthroughs for CVD and T1D.
What we’re saying
“Cardiovascular disease remains the leading cause of death for people with type 1 diabetes, yet targeted therapies and guidelines are still lacking. In collaboration with IDF Europe, ISPAD, and aligned with the European Commission’s cardiovascular plan, this event brings together researchers, clinicians, patient advocates, regulators, and industry partners to drive collaborative solutions and accelerate progress toward better prevention and treatment strategies.”
Addressing the challenge of cardiovascular complications is a cornerstone of our Improving Lives strategy. Breakthrough T1D is actively supporting clinical trials for therapies that can reduce or prevent CVD in people with T1D, and we are working on identifying new projects and researchers to fund to further accelerate these efforts.
We are committed—on a global scale—to making sure people with T1D live the best lives possible, which includes having accessible therapies that reduce the risk of heart complications. Events like these bring together the right people that can make this happen in Europe and beyond.
Bonjour from Montreal, Canada, where the latest and greatest in type 1 diabetes (T1D) research was presented at the International Society for Pediatric and Adolescent Diabetes (ISPAD) 51st Annual Conference. ISPAD is a yearly highlight of the T1D conference calendar, and this year was no exception. Scientists, clinicians, researchers, industry members, people with diabetes, and more were on hand to provide updates in cures, improving lives, access, and more—with much of it being supported by Breakthrough T1D.
Let’s take a look at the highlights.
About ISPAD

ISPAD is one of Breakthrough T1D’s peer organizations. They are dedicated to childhood and adolescent diabetes all over the world. In addition to collaborating in scientific advancements, Breakthrough T1D and ISPAD also fund several grants together—including providing support for clinical fellows from around the world to learn how to care for people with T1D from top-tier clinicians.
Tzield is being studied in kids under 8
Tzield has been in the news for a variety of reasons lately. Sanofi—the manufacturer of Tzield—recently received a voucher from the FDA for the use of Tzield in symptomatic, stage 3 T1D (for which it is not yet approved), potentially expediting the regulatory review process.
Tzield is currently approved for children and adults aged eight years and older in stage 2 T1D to delay the onset of stage 3 T1D. An ongoing study is investigating if Tzield can delay the progression of T1D from stage 2 to stage 3 in a new demographic: kids under eight.
At the ISPAD conference, researchers presented an interim analysis from the PETITE-T1D study, which is investigating if Tzield is as safe and tolerable in kids under eight in stage 2 T1D as it is in older individuals.
So far, 23 children have received a 14-day course of Tzield. To date, the safety and tolerability profile of Tzield is similar to that of older individuals who receive the drug. Two children in the study have progressed to stage 3 T1D.
This study is ongoing and we look forward to seeing more data.
Key takeaway
Tzield can delay T1D onset by an average of 3 years. This important study is determining if a new population—kids with T1D under eight years old—can benefit, and the data so far is encouraging.
Breakthrough T1D taking the stage

Breakthrough T1D leadership was on site to present and chair sessions.
- Chief Scientific Officer Sanjoy Dutta, Ph.D., gave an overview of Breakthrough TD’s mission strategy and chaired a session jointly with ISPAD.
- Chief Medical Officer, Global, Thomas Danne, M.D., was there to discuss Time in Normoglycemia, which is blood sugars between 70 and 140 mg/dl, and how technology can help kids achieve it.
- Vice President of Medical Affairs, Anastasia Albanese-O’Neill, Ph.D., APRN, CDCES, served as faculty for the 21st Annual ISPAD Science School for Health Professionals that took place immediately before the ISPAD Conference.
- Community Engagement & Communications Director, Global Access & Global Responsibility, Renza Scibilia, hosted a symposium on advancing diabetes care in complex settings.
Technology improves outcomes
A motif that was repeated by many, many investigators: automated insulin delivery (AID) systems help people do better. This spans several different technologies and systems: Tandem Control IQ, Minimed 780G, Cam APS, and OmniPod. Here’s why:
- The near-constant adjustments made by these systems are always working to meet glycemic targets.
- They can help people get closer to their glycemic targets with less user input.
- They lower the mental burden of T1D, helping people less engaged with managing their T1D do better.
Key takeaway
It is widely established that AID systems lead to better outcomes. It’s important to continue to ensure that everyone has access to these systems and that they work better with less user input. They must become the standard of care across the world for kids with T1D.
The importance of clinical trials for stage 3 T1D
Stage 3 is when people are typically diagnosed with T1D. At this stage, beta cell function has not been completely eliminated. Individuals are still making insulin—not enough, but some. Decades of research by Breakthrough T1D and others has demonstrated clear benefits to holding onto beta cell function for as long as possible.
Clinical trials for individuals in stage 3 are few and far between. While there are exceptions (like the recently announced BARICADE-PRESERVE), we need more trials because people in stage 3 T1D can benefit from therapies to keep these cells alive and producing insulin for longer.
Key takeaway
Prioritizing clinical trials for stage 3 T1D—and recruiting for them—can be a bridge between better glycemic control today and future cures.
See you in 2026

2026 is going to be a big year—with many more T1D conferences to cover. This includes, for the first time, our very own conference: the 2026 Breakthrough T1D Clinical and Research Congress.
Stay tuned for the 2025 Top Advances piece in the coming weeks to see everything we’ve accomplished this year as we look towards the next one.
To drive our mission forward, Breakthrough T1D financially supports type 1 diabetes (T1D) research through training awards and grants to academic scientists or clinicians, investments in promising companies, conference support, and more. Here, we take a deeper dive specifically into our training awards offered to early-stage researchers to understand the impact and outcomes that these awards have on individual careers and T1D research as a whole.
Key Takeaways
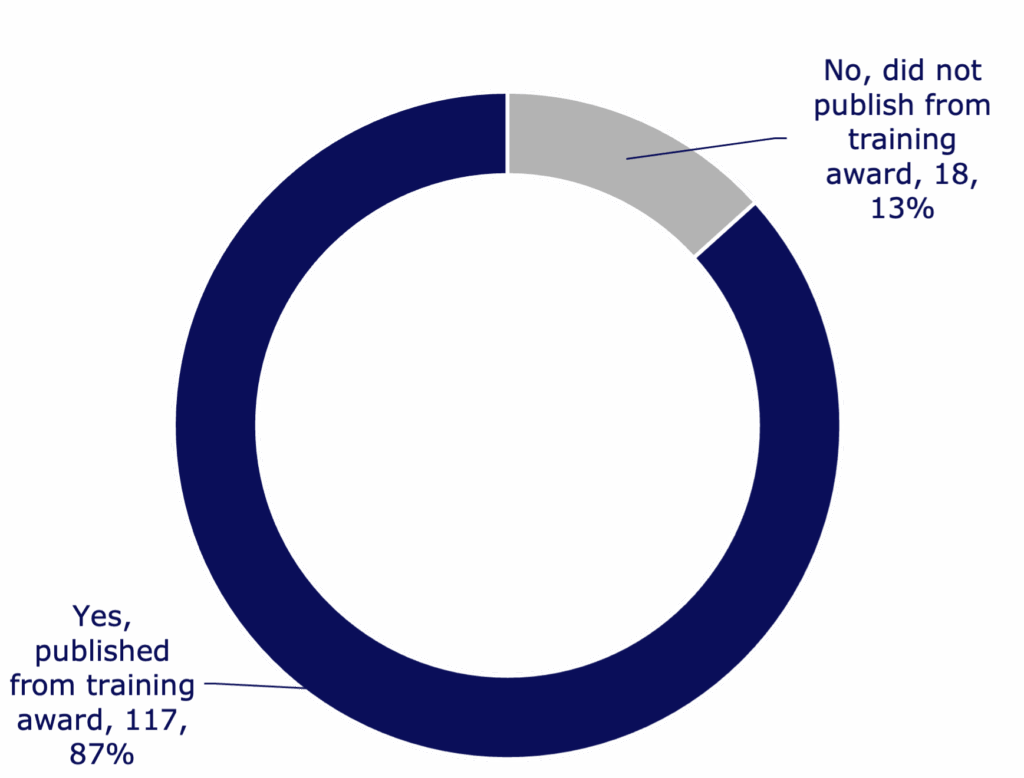
- Breakthrough T1D conducted a survey of researchers and clinicians who received training awards from 2015 to 2024, totaling $62,513,719 in funding.
- The purpose was to understand the impact of the training awards on career decisions and the research and clinical outcomes stemming from the awards.
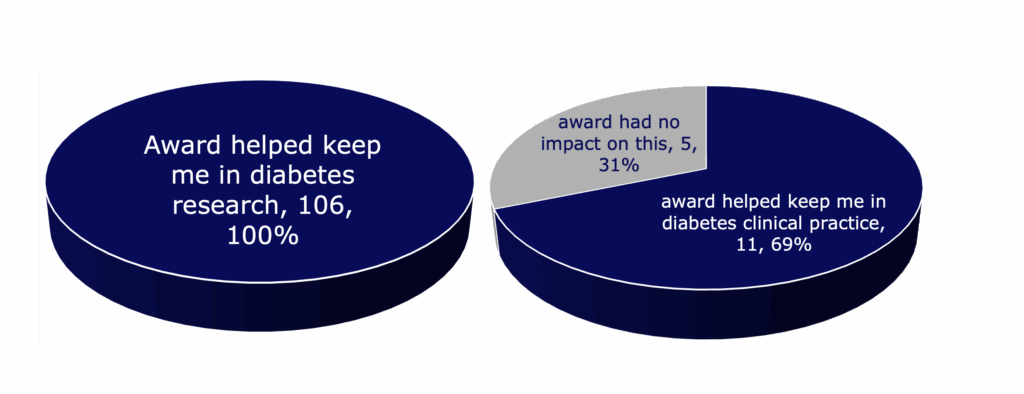
- Most Breakthrough T1D award recipients stayed in diabetes research or clinical practice after the award ended.
- Training award recipients reported publications, patents, honors, clinical trials, collaborations, resources, and mentoring of new trainees arising from Breakthrough T1D training awards.
- Recipients gave positive feedback on the awards, and many continue to be involved in Breakthrough T1D beyond applying for funding.
Why did we conduct this survey?
Training awards are a key part of Breakthrough T1D’s grants portfolio. These awards support early-career scientists and clinical researchers to explore promising ideas and encourage trainees to follow career paths that drive T1D research forward. Our approach is designed to provide opportunities to support trainees from an early postdoctoral stage (after receiving a higher degree) to transitioning to an independent lab and career as a T1D researcher.
Breakthrough T1D previously conducted surveys analyzing the impact and outcomes of training awards completed between 1974 to 2014, revealing that these awards encouraged long-term pursuit of a career in T1D. The current survey included an expanded analysis on training awards completed between 2015 to 2024. The results provide a broader understanding of how our financial support impacts research outcomes, career paths, and additional funding secured by awardees. This way, we can ensure that our funds for trainees are being used in the best way possible, supporting the best and brightest researchers and ideas that drive our mission forward now and in the future.
Survey methodology
From 2015 to 2024…
$62,513,719
Dollar amount of Breakthrough T1D funds invested in training awards
269
Number of training awards granted
230
Number of unique award recipients
Types of training awards surveyed
| Training award | Number of awards granted between 2015-2024 | Number of awards evaluated based on survey responses |
|---|---|---|
| Postdoctoral Fellowship | 155 | 80 |
| Advanced Postdoctoral Fellowship | 48 | 35 |
| Career Development Awards | 36 | 26 |
| Kellogg Family Early Career Patient-Oriented Diabetes Research Awards | 8 | 4 |
| Transition Awards | 22 | 17 |
| Total | 269 | 162 |
Of the total training awards funded between 2015 to 2024, this survey is a reflection of 135 respondents (60%) covering 162 awards. Respondent data was analyzed, mapped, and reported.
Impacts on career trajectories
17
Number of countries where respondents are located
76%
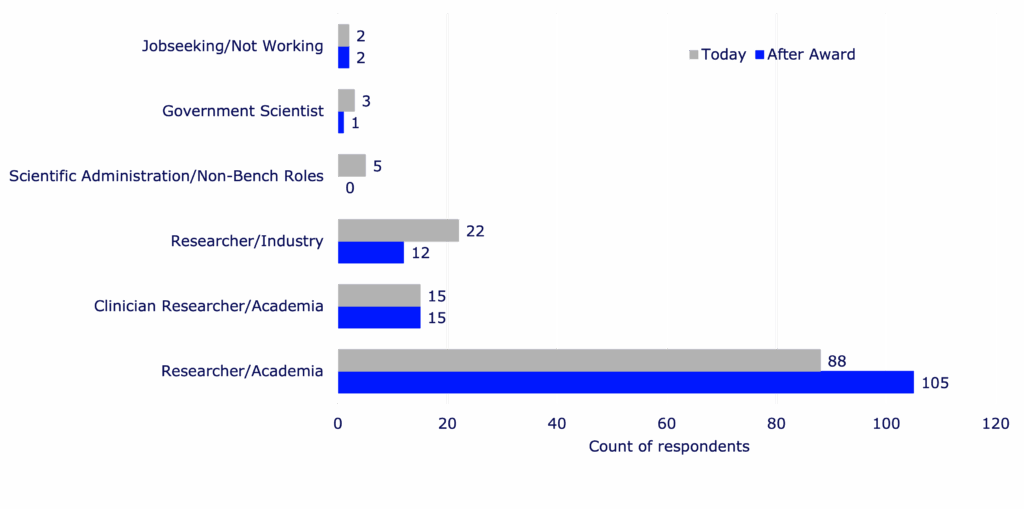
Percent of respondents who remained in academia after award completion
79%
Percent of respondents still working in diabetes (including T1D and type 2 diabetes) in both research and/or clinical practice
Essential findings: Career trajectories for training award recipients
- Researchers take on more senior positions post-Breakthrough T1D award, including tenure-track roles.
- Every respondent working in diabetes research reported that the training award influenced their decision to continue to focus on diabetes.
- Those who received more advanced training awards were more likely to stay in the diabetes field.
- The main reasons researchers and clinicians stayed in diabetes are that the awards support training, networking, and publications.
- If given the option, most respondents chose the Breakthrough T1D training award over other training award options, primarily due to prestige and better award terms.
Impacts on research outcomes
Research supported by training awards resulted in a variety of outcomes that could benefit the larger scientific community and drive T1D research forward. Readouts of successful research include peer-reviewed journal publications, clinical trials, patents, collaborations, resources, grant review requests, and research honors or recognitions.
463
Number of published papers reported by respondents
176
Number of journals that respondents published in
41
Number of patent-related activities reported from training awards
57%
Percent of respondents who formed at least one and often multiple collaborations throughout the training award
57
Number of different shareable resources developed by award recipients
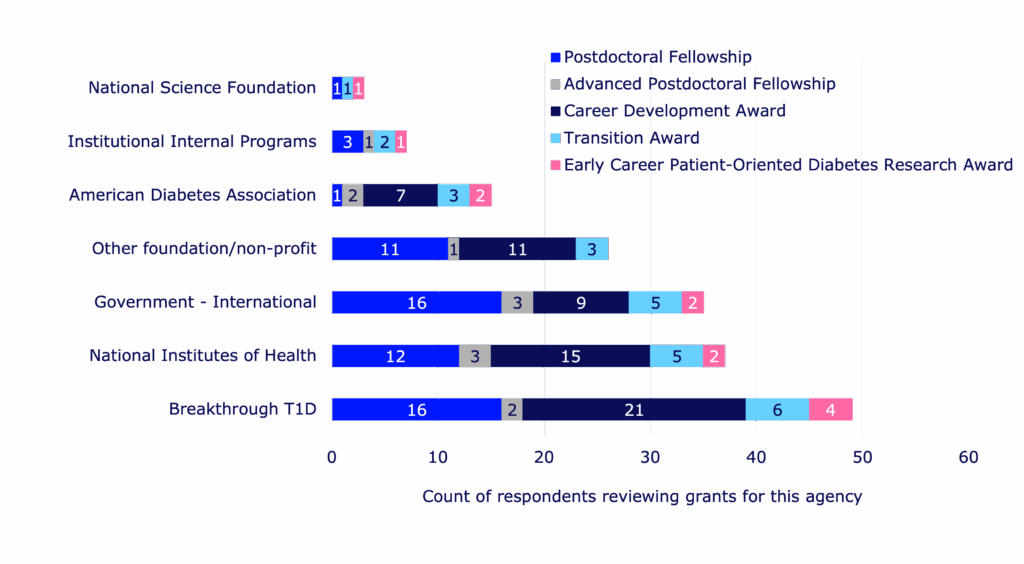
39%
Percent of respondents who serve as grant reviewers for Breakthrough T1D
31
Percent of respondents who reported receiving at least one research honor
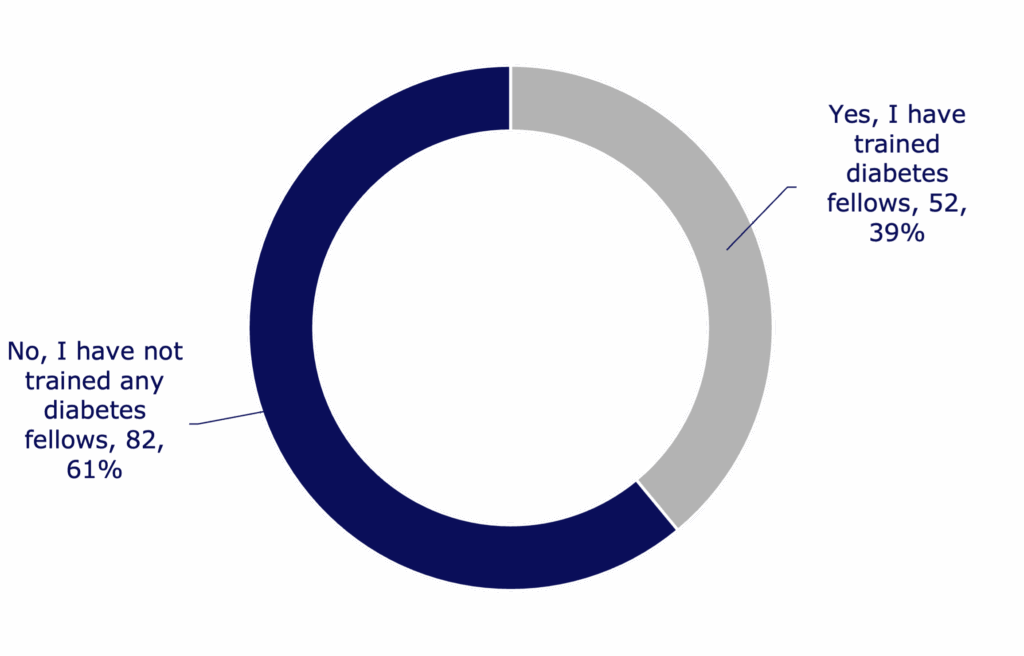
91
Total number of research fellows trained by respondents
84%
Percent of next-generation trainees in academic research or clinical practice
Essential findings: Research outcomes stemming from awards
- Respondents from every single type of training award report having trained a next-generation diabetes research fellow.
- Recipients of Career Development Awards published the most papers and formed the most collaborations.
- Recipients of Postdoctoral Fellowships were the least likely to form a collaboration, revealing a potential opportunity for Breakthrough T1D to help facilitate more collaborative connections amongst this awardee group.
- Awardees reported holding various professional roles in the diabetes community, including consultants to nonprofits and industry, conference planners, program leaders, and manuscript reviewers.
- The most prominent honor reported was a recognition or excellence award.
Impacts on securing additional funding
Breakthrough T1D training awards help early-career researchers continue an engaging and successful career in diabetes research. This is an important factor in securing further funding after the awards end, which helps researchers and clinicians maintain the ability to continue critical research that helps drive our mission forward.
78%
Percent of respondents who reported receiving additional research funding since the completion of the training award
$327,880,689
Dollar amount of additional research funding secured by respondents from various funding sources
Essential findings: Additional funds secured for research
- For each training award, recipients secured at least five times the training award amount in additional funding post-award.
- Postdoctoral fellows exhibited the greatest return on investment—meaning they secured the greatest amount of additional funding after the award ended.
Overall impacts on early career development

Essential findings: Impact on early career development as a whole
- Early-stage trainees prioritize the ability to test a promising research idea that others would not support.
- Later-stage trainees prioritize getting promotion or tenure and generating data to secure additional research funding.
Training awards are key to achieving our mission
Based on these data, it’s clear that Breakthrough T1D’s training awards impact early-career researchers and clinicians across the board: from career development, to training the next generation, to securing additional research funding, to publishing more papers.
This is exemplified by feedback from trainees. Some reported that Breakthrough T1D’s training awards are the best in their class and are critical for T1D research, including the ability to execute niche projects that other funding agencies would not support. Most respondents reported being immensely grateful for their training awards. See what some of the awardees have to say:

“Unequivocally, the award launched my career as a diabetes researcher.”
–Danielle Dean, Ph.D., Postdoctoral Fellowship Award recipient
“I am now fortunate to have mentees below me (one of whom received a Career Development Award). So not only did this award help me, but it helped establish an enduring source for new trainees interested in diabetes research.”
–Jeremy Pettus, M.D., Kellogg Family Early Career Patient-Oriented Diabetes Research Award recipient


“[I have made] International collaborations … research institutions and biotech companies to advance therapy development… partnerships across Europe, Asia, and the U.S.”
–Luis Arnes, Ph.D., Advanced Post Doctoral Fellowship and Transition Award recipient
Not only do these awards help retain talent in diabetes research, but they also help awardees maintain connections with Breakthrough T1D as a whole—outside of seeking financial support. Training award recipients have presented research updates at local events, participated in Breakthrough T1D fundraising activities, and supported our advocacy efforts. We are helping to build the next generation of T1D researchers, advocates, T1D community supporters, and more—all working toward the same goal of a world without T1D.
This survey has put into context where our money is going—and it’s going to the right places. Helping to train the next generation of scientists and clinicians is key to the success of our mission. We’re seeing significant returns on our investments, embodied by trainees securing substantial funding for ongoing T1D research, the creation of shareable resources that will help drive research forward and increase collaboration, and the success of our awardees in securing desirable jobs and leadership positions.
We put our time and money into identifying the best-and-brightest scientists and ideas, encourage them to stay in the field, and guide them as they pass the torch to the upcoming talented pool of young T1D researchers. We support scientists so they can do the important work that will help people with T1D live better lives—and maybe one day live without T1D. And, most importantly, we wouldn’t be able to do this without YOU: our supporters, our donors, and the entire T1D community that allows us to do what we do every day—improve the lives of people with T1D as we drive towards a cure. Thank you.
The full-length report was put together with the assistance of Breakthrough T1D consultant Kim Hunter-Schaedle.
There is always breaking, inspiring news in the world of type 1 diabetes (T1D) research—and that news was front and center at the 2025 European Association for the Study of Diabetes (EASD) Meeting.
This annual event is one of the largest diabetes conferences in the world, and Breakthrough T1D staff, partners, funded researchers, and more were there to participate, share new data, and stay current on the state of T1D and the path to cures.
At EASD 2025, we heard the best and the brightest in T1D present on every area of our Mission priorities, from advances in cell therapies to building consensus around general population screening for T1D.
Check out the highlights below!
Long-awaited updates on two disease-modifying therapies: ATG and verapamil
Excitingly, the results from the MELD-ATG and Ver-A-T1D clinical trials were presented at EASD, co-chaired by our very own Sanjoy Dutta, Ph.D., Chief Scientific Officer at Breakthrough T1D. Each of these studies were made possible through a collaboration between INNODIA, Breakthrough T1D, the Helmsley Charitable Trust, industry, the European Commission’s Innovative Medicines/Health Initiative (IMI/IHI), and investigators around the world. Each of these studies incorporated the INNODIA master protocol, an effort to align on clinical trial design and evaluation for investigational therapies for newly diagnosed T1D.
The phase 2 MELD-ATG study, presented by Chantal Mathieu, M.D., Ph.D., investigated whether a minimal low dose of anti-thymocyte globulin (ATG) could preserve beta cells in children, adolescents, and young adults (5-25 years old) newly diagnosed with clinical stage 3 T1D. ATG works by blocking the immune cells that destroy beta cells. Here’s a summary of the recently published data:
- Using an adaptive clinical trial design, the study examined multiple doses of ATG at once, eventually moving forward with 0.5 mg/kg and 2.5 mg/kg in comparison to placebo.
- The minimal effective low dose for ATG is 0.5 mg/kg. This dose had fewer side effects compared to the higher doses, and it was generally well-tolerated.
- Participants who received low dose ATG demonstrated clinically significant higher C-peptide levels during the treatment period compared to placebo. This was accompanied by lower HbA1c levels.
The phase 2 Ver-A-T1D study, presented by Thomas Pieber, M.D., investigated whether verapamil could preserve beta cells in adults (18-44 years old) with newly diagnosed stage 3 T1D. Verapamil is a blood pressure medication that may also reduce beta cell stress, and initial clinical studies suggest that it may protect beta cells in newly diagnosed T1D. The key findings presented are:
- At 12 months, there was no statistically meaningful difference in C-peptide levels between those who were treated with verapamil versus placebo. This was accompanied by no differences in insulin dose or continuous glucose monitor (CGM) metrics. There was a trend toward better C-peptide preservation in the treatment arm compared to placebo but missed statistical significance marginally.
- Verapamil was generally safe and well-tolerated, with no unexpected adverse events but some reported mild and reversible cardiac disorders.
- The study investigators concluded that future studies testing verapamil in T1D require a larger population and longer duration—and that verapamil may be a candidate to combine with immunomodulatory disease-modifying therapies.
- The Ver-A-Long extension study will follow people who continued with verapamil treatment after this trial ended.
Key takeaways
The horizon is bright for ATG—investigators have now identified the lowest dose needed to preserve beta cells in newly diagnosed children and young adults with T1D, with the fewest side effects possible. BT1D and the T1D Fund are supporting the development of a next generation ATG with a manageable/lower side effect profile compared to the medication used in the current trial. On the other hand, the future is open for verapamil—more studies are needed to determine if it can delay beta cell loss and T1D progression. A biotechnology company, TiXiMed, is developing a next-generation verapamil (TX-100), with a more favorable pharmacological profile.
Emerging consensus for screening the general population for T1D
Breakthrough T1D spotlight: Early detection and screening
Breakthrough T1D’s Vice President of Medical Affairs, Anastasia Albanese-O’Neill, Ph.D., APRN, CDCES, spearheaded an effort to assemble a working group of nearly 30 experts to establish a consensus on T1D screening guidance. This session—which Dr. Albanese-O’Neill chaired—included presentations by some of these experts, who gave the audience a sneak peek on how to integrate population-level screening for T1D into clinical practice.
In this session, Marian Rewers, M.D., Ph.D., Anette Ziegler, M.D.,and Chantal Mathieu, M.D., Ph.D., provided an overview of soon-to-be-published consensus screening guidance for the general population. These presentations highlighted an urgent need for population-level screening given the rising global incidence of T1D and the known benefits of early detection, including time to prepare and potentially delay disease progression.
T1D autoantibodies
Autoantibodies are proteins that may signal that the body’s immune system is attacking insulin-producing cells in the pancreas, and they can easily be measured by a blood test. If a person has two or more persistent autoantibodies, it’s very likely they’ll develop T1D.
The presenters discussed benefits, harms, and methods of T1D screening; who should be screened and how often; and how to effectively communicate screening results. Some notable takeaways:
- Early detection of T1D—prior to clinical onset and at the time of diagnosis or later—has enormous benefits, including prevention of diabetic ketoacidosis, opportunities to delay progression with approved therapies or experimental therapies in clinical trials, early control of hyperglycemia that can reduce the risk of complications, and more.
- Providing support during the screening process will be important, such as mitigating pain associated with obtaining blood samples or reducing anxiety.
- Every initial screening result must be confirmed with another test before diagnosis.
- Infrastructure and policy must be in place before population-level screening can be implemented into clinical practice, including established processes for confirming positive test results, monitoring people with diagnosed early-stage T1D, and referring people to specialists.
- General population screening is suggested starting at 2-4 years old, and again at 6-8 years old and 10-15 years old if negative for autoantibodies.
- Healthcare providers (HCPs) need to be knowledgeable on key aspects of the screening process—and be able to effectively communicate what screening results mean.


Breakthrough T1D spotlight: Pediatric screening for T1D
To increase pediatric T1D screening in the UK, Breakthrough T1D is co-funding the ELSA (EarLy Surveillance of Autoimmune disease) study with Diabetes UK. At EASD, Parth Narendran, FRCP, Ph.D., presented how the data from this study was used to assess the acceptability and feasibility of general population screening for presymptomatic T1D in children who are 3-13 years old. The research team found that an initial finger-prick dried blood spot test—which can be performed at home or school and mailed to the team—was acceptable as an initial autoantibody screen, followed by a confirmatory blood test. Most families found out about this screening opportunity through social media, school, or their general practitioner—and none regretted participating, regardless of the outcome. This study will continue with an expanded population of children who are 2-17 years old.
Key takeaways
Consensus guidance from T1D experts suggests that population-level screening for T1D, starting at a young age, will be critically important for detecting T1D early so families have ample time to prepare and potentially delay disease progression. This consensus guidance will soon be published in a peer-reviewed journal, with the goal of integrating T1D screening into clinical practice. Breakthrough T1D is committed to expanding T1D early detection on a global scale.
Cell therapies are coming
Cell Therapies replace destroyed beta cells with external cells that make insulin and protect them so that they can function for a very long time. It’s one of our primary strategies to achieve cures for T1D—as exemplified by Project Accelerate Cell Therapies.
Our goal is to grow these cells in a laboratory and discover a way to keep them safe inside the body without the need for chronic immunosuppression. EASD provided several reminders that these therapies are not science fiction—they’re working in clinical trials, and we must continue to push these first-generation therapies—and future generations—across the finish line.
Vertex and zimislecel
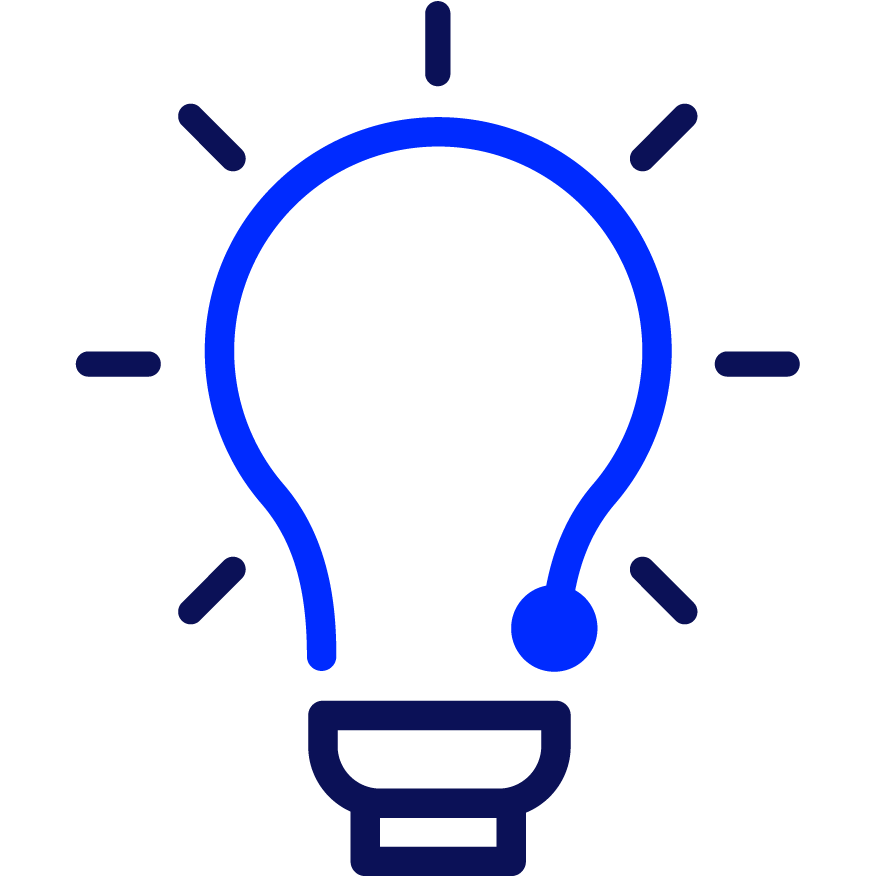
Attendees at EASD were treated to another presentation demonstrating the efficacy of Vertex’s zimislecel, a manufactured islet cell replacement therapy. Michiel Nijhoff, M.D., presented some highlights from the FORWARD study, all of which were previously presented at other diabetes conferences and published in the New England Journal of Medicine [subscription required] this summer.
This chart does an excellent job illustrating the drastic improvement in glucose control in the year following transplantation of the cells. As does this stat: the 12 individuals in the first phase of the study went from 55.5% time in range and 36.1 units of insulin per day at the start of the study to 95% time in range and 0 units of insulin per day after one year.
Breakthrough T1D’s support for Doug Melton, Ph.D.—whose proprietary lab-created beta cells are now being advanced by Vertex—goes back decades, both via research grants and an investment from the T1D Fund: A Breakthrough T1D Venture. We can’t wait to see more data when the Phase 1/2/3 trial is completed in the coming months.
Multiple strategies moving forward
Several other investigators shared data on their approaches to bringing scalable cell therapies to people with T1D. This includes:
- Matthis Hebrok, Ph.D., a longtime Breakthrough T1D collaborator, showed his research on hypoimmune, gene-edited islets. These cells are genetically engineered to avoid the immune system.
- Maria Nostro, Ph.D., another Breakthrough T1D-funded researcher, is using a different protection strategy: macrophages. Macrophages are a type of white blood cell that we have learned are present in the developing pancreas. The idea here is that we can implant the macrophages alongside the beta cells during islet transplant and it will help protect and vascularize the cells. This research is still pre-clinical but represents another “shot on goal” to keep these cells safe.
- Orizuru Therapeutics (a T1D Fund Company) presented on OZTx-410, an investigational cell therapy composed of human manufactured islets derived from adult cells that were reprogrammed to precursor cells and eventually islets. This is one of the first cell therapies for T1D derived from reprogrammed adult cells, and this therapy is suitable for manufacturing at large scale. Earlier this year, these cells were implanted into a person with T1D as multiple “sheets” in the abdominal wall. Orizuru expects to share results in the coming months.
- Allarta, a company with Breakthrough T1D funding, shared pre-clinical data on their hydrogel, which allows islet transplants without the use of immunosuppression. Their product is showing efficacy in animal models, and they are progressing towards human clinical studies.
Breakthrough T1D spotlight: Personal journeys and emerging T1D therapies
Breakthrough T1D’s Chief of Global Medical Affairs, Thomas Danne, M.D., Ph.D., hosted an interactive workshop for participants to better understand the personal journeys of people with T1D through videos from T1D community members and their families. Dr. Danne—along with other Breakthrough T1D staff and in conjunction with Eelco J.P. de Koning, M.D., Ph.D., and Valera Sordi, Ph.D.—fielded questions from the audience and covered topics spanning immunosuppression, how islets are derived from donor pancreases, T1D screening, and more.


Key takeaways
Cell therapies are real and advancing, with promising results in clinical trials, including dramatic improvements in glucose control. Also moving forward are ways to keep them safe and happy without the use of immunosuppressants.
Continuous ketone monitoring is coming – for good reason!
Diabetic ketoacidosis (DKA) is a serious and, unfortunately, too-common complication of life with T1D. This dangerous condition happens when the body does not have enough insulin. DKA is life-threatening and costly.
Breakthrough T1D-funded research Jennifer Sherr, M.D., Ph.D., presented this, outlining that this is a problem and that there is room for improvement in identifying DKA and agreeing on the interventions. We need better mitigation strategies, and if we get them, people will do better.

Breakthrough T1D Medical Affairs: CKM
Breakthrough T1D has prioritized the development of continuous ketone monitors, which we believe can be lifesaving. Our Medical Affairs team is working to build consensus around these and how they can be useful in the clinic and in the lives of people with T1D.
Key takeaways
Peter Bergstal gave a great summary, where he noted the CKM can be incredibly useful if we hit a few key checkpoints. These include developing an accurate CKM, receiving regulatory approval, finding the people most likely to benefit, and they’re covered by payers.
T1D Index 3.0 is here
The T1D Index—a computational tool launched in 2022 that measures the human, public health, and economic impact of T1D around the world—was developed by Breakthrough T1D in collaboration with Life for a Child, the International Diabetes Foundation (IDF), and the International Society for Pediatric and Adolescent Diabetes (ISPAD). Renza Scibilia, part of the Global Responsibility team at Breakthrough T1D, presented about exciting new upgrades for the T1D Index version 3.0. Some important updates:
- The newest version of the T1D Index has been updated with new published and unpublished data to ensure it remains the most comprehensive picture of T1D in the world.
- The new-and-improved dashboard allows for easier usability for researchers, healthcare professionals, and T1D community members to explore the data and computationally model the effects of care and diagnosis.
- The next version of the T1D Index in the works will further break down T1D statistics, focusing on regions and states within countries.
- The T1D Index is critically important for global advocacy and collaboration efforts by providing evidentiary data that support initiatives to change the trajectory of T1D.
9.5 million
The number of estimated people living with T1D across the globe.
1.5 million
The number of people at all ages living with T1D in the United States—the country with the highest prevalence of T1D.
Key takeaways
The T1D Index 3.0 is the most updated, accurate version yet, offering the best possible global coverage of T1D statistics and an enhanced dashboard to simulate changes in T1D incidence and prevalence.
The path to fully closed-loop automated insulin delivery systems
Hybrid closed-loop automated insulin delivery (AID) systems—a continuous glucose monitor (CGM) paired with an insulin pump, requiring mealtime and physical activity announcements—are estimated to be used by hundreds of thousands of people today. While these devices have been transformative for the T1D community, user input is still a daily burden, and fully closed-loop systems aren’t widely available for T1D yet. In this session, Moshe Phillip, M.D., Katrien Benhalima, M.D., Ph.D., and Charlotte Boughton, M.D., Ph.D., explored the benefits of currently available AID systems and fully closed-loop AID systems on the horizon.
- Based on clinical trial data and real-world evidence, hybrid closed-loop systems improve time-in-range (TIR) and reduce HbA1c levels without increasing the risk of hypoglycemia. This translates to better outcomes and quality of life for people with T1D.
- Different AID systems will work better for different people, depending on preferences for blood glucose management. Because there have been limited head-to-head trials comparing two or more AID systems, there is no evidence that any one system is definitively better than another.
- Right now, only one AID system is licensed for use in pregnancy (CamAPS FX). More research is needed to determine safety and efficacy of additional AID systems for use during pregnancy, and real-world evidence of women who become pregnant while using different AID systems may provide some clues.
- A clinical trial for the CamAPS HX fully closed-loop AID system in the U.K. increased TIR by 50% compared to 36% in people using standard pump therapy with a CGM—amounting to three additional hours each day of blood glucose in target range. Participants reported improved mood and sleep, less stress, and reduced diabetes burden. Similar improvements in blood glucose and daily life were reported in adolescents.
- Next-generation AID systems under investigation include two or more hormones for optimal glycemic control, artificial intelligence, and “digital twin” simulation tools.
Key takeaways
Fully closed-loop AID systems are coming, but they’re not here yet. Initial clinical studies using fully closed-loop systems in T1D demonstrate significant improvements in blood glucose control and quality of life. In the meantime, hybrid closed-loop systems offer substantial benefits for people with T1D—and there are many commercially available systems to choose from based on individual preferences.
Updates on heart and eye complications
Reducing T1D complications remains a major research focus area around the globe. Robert Humphrey presented on the LENS trial, which found that fenofibrate—a generic and affordable cholesterol-lowering medication—can reduce the progression of diabetic retinopathy in people with T1D and type 2 diabetes. A follow-up study found that fenofibrate is a cost-effective option for diabetic retinopathy in the U.K., especially for T1D. In conjunction with these studies, the recruiting Breakthrough T1D-funded Protocol AF trial is further investigating fenofibrate in preventing progression of diabetic retinopathy in people with T1D.
Rebecka Bergdal presented on a study examining the relationship between cumulative exposure to glycemia or lipids and heart failure, one of the leading causes of cardiovascular mortality in T1D. The study found that both cumulative glycemic exposure (defined as time spent with HbA1c levels > 7%) and cumulative lipid exposure (LDL, triglycerides, and cholesterol) are independently associated with increased risk for heart failure. The publication highlighting these findings includes a call to action for healthcare providers to help people with T1D minimize high blood sugar and lipid exposure, and support and encourage the best diabetes management possible to reduce risk for heart complications.
Key takeaways
Exciting clinical trials to prevent worsening of diabetic retinopathy in people with T1D are ongoing. To reduce the risk of heart complications, including heart failure, people with T1D and healthcare providers should aim for minimal exposure to high blood sugar and lipids, and more work is needed to find solutions for cardiovascular disease in T1D.

“We saw significant progress in each area of our Mission at EASD,” said Sanjoy Dutta, Ph.D., Chief Scientific Officer. “The MELD-ATG results are a noteworthy step forward for disease-modifying therapies; the screening consensus is a much-needed stake in ground around general population screening; we are moving closer to urgently needed adjunctive therapies; and cell therapies continue to perform in clinical and pre-clinical studies. It’s an incredibly exciting time in T1D and we will continue to support this work—and more—to bring cures to our community.”
The next big T1D meeting is ISPAD 2025, which will take place November 5-8 in Montreal, Canada. Stay tuned for more news!
This article was written by Sandy Vogt and Brian Herrick.
Since 1994, Marshalls has been a committed supporter of Breakthrough T1D.
As Breakthrough T1D’s largest corporate partner, Marshalls raises more than $3 million each year through in-store fundraisers at checkout, donations from the TJX Foundation, and store support of local Breakthrough T1D chapters across the country. These combined efforts have resulted in more than $47 million in donations to-date for Breakthrough T1D, helping to advance our mission of accelerating life-changing breakthroughs to cure, prevent, and treat type 1 diabetes (T1D) and its complications.
Marshalls’ 2025 in-store fundraising campaign for Breakthrough T1D runs from Sunday, August 31, through Saturday, September 27. Find your local Marshalls here.
Creating a better future

Digital creator Kris Leeper’s type 1 diabetes journey began with his diagnosis in 2013. It was, in his own words, “terrifying.”
“My wife and I were bombarded with conflicting information,” Kris said. “We were navigating unchartered territory as a family, just two years into our marriage. I felt lost and uncertain about the future.”
T1D technology changed the game for Kris. “The introduction of a continuous glucose monitor (CGM) and pump revolutionized my outlook on living with T1D. I immediately felt better,” he said. “Access to and understanding of diabetes technology has profoundly improved my daily life.”
Kris appreciates Marshalls for bringing awareness to Breakthrough T1D’s efforts to make life-changing technologies a reality for the more than 1.5 million Americans facing T1D.
“Companies like Marshalls play a pivotal role in bringing Breakthrough T1D’s mission to the mainstream,” he said. “Marshalls’ support for advocacy, research, and fundraising demonstrates the power of a major brand in creating a better future for people living with or caring for those with T1D.”
Driving breakthroughs forward
1 in every 31 families in the U.S. are impacted by type 1 diabetes—it places a tremendous financial, emotional, and mental burden on those who live with it and their loved ones. With support from partners like Marshalls, Breakthrough T1D is making everyday life with T1D safer and easier through technology—like the CGM Kris uses, and automated insulin delivery systems.
Every donation collected through the Marshalls partnership has also helped drive critical research in cures for type 1 diabetes. From the launch of the Special Diabetes Program in 1997, to the first manufactured islets making insulin in 2014, and the FDA approval of Tzield, the first disease-modifying therapy for type 1 diabetes, Breakthrough T1D is driving innovation forward to make the condition a thing of the past.
“The future of type 1 diabetes is bright,” Kris said. “We are on the cusp of living longer, experiencing fewer complications, and enjoying a life without limits.”
Breakthrough T1D thanks Marshalls for its incredible partnership in improving and changing life with type 1 diabetes.
Hy-Vee’s dedication to Breakthrough T1D’s mission is personal.
Type 1 diabetes (T1D) first struck the Hy-Vee family in 1921, when company co-founder Charles Hyde’s oldest son, Paul, died from the disease when he was 8 years old.
Since 1998, Hy-Vee has been a trusted partner of Breakthrough T1D, raising nearly $20 million through a variety of corporate and store events, including Walks in the Midwestern and Southern U.S. Hy-Vee also participates in Rides across the country; in 2025, they were recognized as a top fundraising team, bringing in over $230,000 for T1D research.
Hy-Vee’s 2025 in-store fundraising campaign for Breakthrough T1D runs from September 1 through 30. Find your local Hy-Vee here.
Working together for cures

Sam and Lauren Raiche are loyal Hy-Vee customers. They’re also dedicated fundraisers, advocates, and volunteers for Breakthrough T1D. Their 8-year-old son, Alexander, was diagnosed with T1D at age 3.
They appreciate Hy-Vee’s commitment to Breakthrough T1D’s mission. “It means so much to know that a company we already trust and shop with is also supporting a mission so close to our hearts,” Lauren said. “Living with type 1 diabetes is a daily challenge for our family, and seeing Hy-Vee stand behind research and programs that directly impact families like ours makes us feel grateful and supported every time we walk through their doors.”
Alexander represented Kansas at the 2025 Breakthrough T1D Children’s Congress. As a Delegate, he met with representatives to advocate for renewal of the Special Diabetes Program, which funds critical type 1 diabetes research. “Advocacy has become a cornerstone of our journey,” Lauren said. “We firmly believe that raising awareness and engaging with the community are essential steps toward making meaningful progress and, ultimately, achieving cures.”
Accelerating cell therapy breakthroughs
Support from partners like Hy-Vee helps fuel that progress toward cures for type 1 diabetes. Breakthrough T1D’s cures portfolio includes cell therapies, which replace destroyed beta cells with protected functional cells to restore insulin therapy independence and glucose control, ideally without immunosuppression.
Over the past decade, Breakthrough T1D has funded more than $156 million in cell therapies research, including partnerships with organizations like Vertex Pharmaceuticals and Sana Biotechnology.
In 2024, Vertex launched a pivotal clinical trial for zimislecel (formerly VX-880), which uses manufactured islets to restore the body’s ability to produce insulin. The therapy, however, requires the use of immunosuppression to protect the transplanted cells from rejection. The islets used in zimislecel are derived from the Breakthrough T1D-funded work of Doug Melton, Ph.D., who first turned precursor cells into insulin-producing cells in 2014.
A 2025 study from Sana Biotechnology showed that hypoimmune (HIP) donor-derived islets are making insulin and avoiding immune detection in the first person treated. The T1D Fund: A Breakthrough T1D Venture invested in Sana to help advance their HIP technology platform.
Breakthrough T1D continues to drive innovation to develop cell replacement therapies and eliminate the need for immunosuppression. With the generosity and support of partners like Hy-Vee and families like the Raiches, we will change the lives of everyone facing type 1 diabetes.
Breakthrough T1D is Wawa’s longest-standing partnership. Since 1994, the popular convenience store chain has raised millions of dollars for type 1 diabetes (T1D) research through in-store fundraising campaigns at more than 1,060 locations across the Eastern U.S.
Wawa’s partnership with Breakthrough T1D is further enhanced by donations from The Wawa Foundation, which is committed to helping Breakthrough T1D create a world without T1D and building strong communities by supporting causes related to health, hunger, and everyday heroes.
Wawa’s 2025 fundraising campaign for Breakthrough T1D runs from July 31 to August 20. Customers can donate $1, $3, or $5 or round up at checkout to support life-changing breakthroughs for people living with T1D.
Wawa lives out the values it speaks to
Wawa super fan, Nate Keeney, has lived with T1D for 27 years. He is proud to support a company that lives out the values it speaks to.
“Long before working at Breakthrough T1D, I loved Wawa for their larger selection of food options and snacks,” Nate said. “I always knew they partnered with the organization, but seeing how their efforts impact everyone living with T1D has given me a renewed passion to continue supporting Wawa.”
Wawa walks to cure T1D
Breakthrough T1D Walk is another way Wawa helps support the T1D community and fund critical T1D research. The Wawa Foundation matches employee Walk fundraising efforts up to $100.
Wawa associates also volunteer at Walk events to distribute in-kind food and beverages to participants.
“I see Wawa everywhere at our local Breakthrough T1D Walk in Philadelphia, with their classic soft pretzels and iced teas (and diet iced teas!) to fuel us as we walk to support T1D research and community,” Nate said. “The Goose also leads the T1D parade before the Walk begins.”
Wawa helps fuel life-changing research
Wawa helps Breakthrough T1D advance its mission to accelerate life-changing breakthroughs to cure, prevent, and treat type 1 diabetes and its complications.
That includes technology like continuous glucose monitors (CGMs) and automated insulin delivery (AID) systems, which have been life-changing for Nate.
“My insulin pump and CGM have been a wonderful addition to my diabetes routine—they’ve helped me get my A1c into a great range and identify trends,” he said. “Also, the ability to manage my T1D from my phone has made life on the go easier, whether it’s traveling for work or traveling for fun to different states or countries.”
Wawa’s support also fuels research in cures for type 1 diabetes. Cell therapies that insert healthy insulin-producing cells into people with T1D with minimal or no immunosuppression is a breakthrough Nate is hopeful for.
“So much effort, intensity, and care are going into funding research, particularly with cell therapies,” he said. “It makes me hopeful for cures within my lifetime.”
Together, Wawa and Breakthrough T1D are championing life-changing research and strengthening the T1D community.
The short version
Breakthrough T1D’s newest publication outlines what the future of beta cell replacement therapies looks like—and how we can make these therapies a reality for everyone with type 1 diabetes (T1D) who wants them through innovative clinical trial design and expanding the pool of eligible trial participants.
Breakthrough T1D, in collaboration with other leading experts in the field, recently published an article titled “Future Directions and Clinical Trial Considerations for Novel Islet β-Cell Replacement Therapies for Type 1 Diabetes” in the journal Diabetes. Breakthrough T1D Research and Advocacy staff who contributed to the publication include Sanjoy Dutta, Ph.D., Chief Scientific Officer, Esther Latres, Ph.D., Vice President of Research, and Marjana Marinac, Pharm.D., Associate Vice President of Regulatory Affairs.
Beta cell replacement therapies have the potential to cure type 1 diabetes (T1D) by removing the need for external insulin. While donor islet transplantation can result in insulin independence and results from early trials with manufactured islets are encouraging, people with T1D need better tools and therapies. This publication serves as a roadmap for the entire field—including researchers, product developers, industry, regulators, clinicians, and people with T1D—to address these needs and ensure that beta cell replacement therapies can get to people with T1D as quickly and safely as possible.
Read on to learn more about what the future of beta cell replacement therapies looks like.
Key Takeaways
- Currently available beta cell replacement therapies are limited to people with T1D with unstable blood sugar management, typically measured by HbA1c levels, in addition to dangerous lows (hypoglycemic events) that require immediate assistance. These therapies also require lifelong immunosuppression.
- New and improved beta cell replacement therapies are on the way, and we need to make sure that they are available for people with T1D beyond those with unstable blood sugar management and severe hypoglycemic events.
- Clinical trials for these therapies must be strategically designed to include more people with T1D given the potential for insulin independence.
- Shared decision-making between people with T1D and their care team will be critical for evaluating the risks versus benefits of a beta cell replacement therapy, especially as more options become available.
Where we are now
People living with T1D depend on external insulin to manage their condition throughout their lives. Despite advancements in diabetes technology, such as continuous glucose monitors (CGMs) and automated insulin delivery (AID) systems, most people with T1D are unable to achieve blood sugar targets, rendering them at a higher risk for complications, reduced quality of life, and lower life expectancy.
It’s simple: we need to do more for the T1D community.
One promising avenue to meet these needs is through cell replacement therapy. Lantidra®, the first FDA-approved donor-derived islet replacement therapy for T1D, has proven to be safe and effective in eliminating severe hypoglycemia, providing insulin independence, and improving quality of life. However, this therapy is limited to people with severe hypoglycemia and requires immunosuppression to prevent rejection of the transplanted cells, which can have side effects. Even more, these donor-derived islets are in limited supply.
Alternative beta cell replacement therapies are emerging—and we can no longer limit their development to people experiencing severe hypoglycemia.
As stated in the publication:
Given the proven benefits of islet transplantation extending far beyond the amelioration of severe hypoglycemia that has been documented and the understanding of the risk profile gained over the past 20 years, consideration must be given to broadening the application for islet beta cell replacement.
Developing cell therapy strategies to meet unmet needs of the T1D population
Breakthrough T1D’s vision for the T1D community includes beta cell replacement therapies with no immunosuppression that are available to everyone who wants them. We are committed to making this a reality through our Project ACT (Accelerate Cell Therapies) initiative, which will accelerate the development of these therapies to achieve our vision as quickly as possible.
Project ACT
Scientific progress takes time, resources, collaborations, and effort. To accelerate islet replacement therapies faster than ever, Breakthrough T1D launched Project ACT (Accelerate Cell Therapies) to simultaneously advance research, development, regulatory policies, access, and adoption of manufactured islet therapies that do not require broad immunosuppression.
We are entering an exciting era of beta cell replacement. Emerging therapies are addressing challenges such as cell source and scalability, resulting in the development of islets derived from sources other than donors, including manufactured islets and porcine islets. Up-and-coming therapies are also testing different transplantation sites, methods of delivery, and cell protection strategies to prevent immune rejection with the fewest side effects possible (and ideally no immunosuppression). Learn more about what scientists are doing to optimize beta cell replacement therapies.
Breakthrough T1D’s continuous support of many of these therapies, such as Vertex’s manufactured islet therapy zimislecel, has been critical to accelerating them through the clinical pipeline. Explore emerging beta cell replacement therapies in clinical trials now—and see how Breakthrough T1D’s commitment to these therapies helped make this possible.
When successful, the advent of new, safe, scalable, and effective beta cell replacement therapies will provide the T1D community with options. As these therapies are moving their way through the pipeline, we need to ensure they are being studied in a broader T1D population who stand to benefit.
So, how do we make this happen?
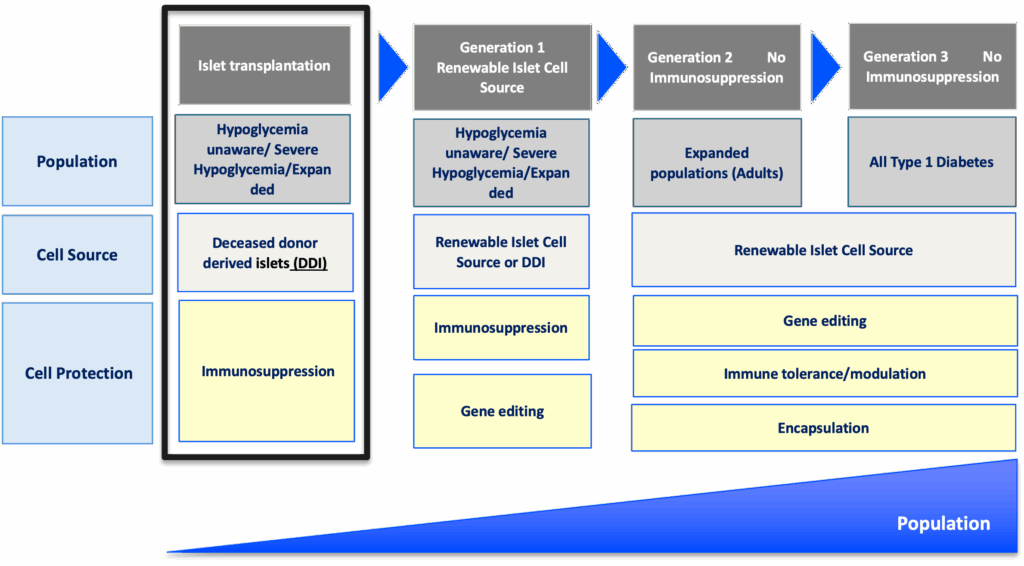
The roadmap for emerging beta cell replacement therapies
The goal
Accelerate availability of emerging next-generation beta cell replacement therapies for every person with T1D who wants them by designing clinical trials that speed their development, regulatory approval, access, and adoption.
Expanding the T1D population eligible for beta cell transplantation
Current trials testing beta cell therapies necessitating immunosuppression require participants to have elevated HbA1c levels and recurrent severe hypoglycemic events. This limits the pool of participants to people who meet the requirements and are most likely to benefit, given the side effects associated with chronic, broad immunosuppressants.
Clinical trials for emerging beta cell replacement therapies should broaden eligibility criteria so more people with T1D can participate—and experience the potential benefits. When designing new clinical trials, sponsors and regulators should consider including a broader range of HbA1c levels, clinically important or serious hypoglycemic events, and other complications.
Studies have found that the T1D community is generally open to beta cell replacement therapies as a potential solution to T1D, and people are willing to accept the associated risk versus benefit considerations for the possibility of becoming insulin independent. A Breakthrough T1D assessment also found that physicians are interested in recommending beta cell replacement therapies to people with T1D—especially if they don’t require immunosuppression.
“Clinical trials to support the development of islet cell replacement therapies need to evolve to include a broader representation of people living with T1D who could benefit from these novel therapies. This includes expanding the outcomes used to assess the benefits of cell replacement that reflect how people with T1D feel and function.”
Marjana Marinac, Pharm.D., Associate Vice President of Regulatory Affairs

Placing people with T1D at the center of clinical trial design
The outcomes used to assess the effectiveness of cell therapies currently in clinical trials, including those involving deceased donor islets, are acceptable for emerging beta cell replacement therapies. These include on-target HbA1c levels, absence of severe hypoglycemia, significant reduction or elimination of external insulin, and restoration of the body’s insulin production as measured by C-peptide.
Other endpoints that should be considered include CGM metric targets like time-in-range in addition to person-reported outcomes. Understanding how a beta cell therapy may affect a person’s health-related quality of life—such as diabetes distress, fear of hypoglycemia, or social and family dynamics—will be critically important for calculating the risk to benefit ratio of these therapies.
Read more about why person-reported outcomes are important for cell therapies.

“There are still significant unmet needs in the T1D community. Breakthrough T1D’s roadmap is supported by the assessment of clinically meaningful outcomes and driving research toward solutions that address key factors such as cell sources and protections strategies that will broaden the people with T1D who could benefit from emerging cell replacement therapies.”
Esther Latres, Ph.D., Vice President of Research
Innovative trial designs to accelerate development of cell therapies
Clinical trials for beta cell replacement therapies are generally based on a single-arm, open-label design—meaning there is no placebo group and both participants and researchers know which therapy is being administered. While this design can work for emerging beta cell therapies, single trials with multiple arms testing alternative transplant sites, immune protection strategies, or other methods have the potential to speed up the pace of development.
Similarly, adaptive trial designs use mid-trial interim analyses of study data to inform the remainder of the trial. This helps researchers learn what’s working (or not working) and adjust the design accordingly, with guidance from regulatory agencies, so the rest of the trial is a focused and efficient use of time and resources. Potential interim changes to trial design include reducing the number of participants required, eliminating doses, recruiting people who are most likely to benefit, or stopping the trial outright due to clear success or failure.
By applying guidance in therapeutic development and innovative trial designs to emerging beta cell replacement therapies, we can move early-stage trials along faster, thereby allowing regulators to make decisions sooner. To support quicker trials and reduce the possibility for delays, researchers, developers, and regulators around the world need to work together to achieve convergence on trial populations, endpoints, and innovative designs that will meet regional requirements.
Learning from past successes
People with T1D continue to live with unmet needs with still significant risk for long-term complications, and they need more therapeutic options. Right now, most clinical trials for beta cell replacement therapies requiring immunosuppression are limited to a small portion of the T1D population. This needs to change—especially given the potential for insulin independence. The T1D community must be put first when making decisions about beta cell replacement therapies, and Breakthrough T1D is making sure that this happens.
Adjusting how we approach clinical trials for emerging beta cell replacement therapies will be critical for ensuring we accelerate the research, development, regulatory approval, access and adoption of these novel therapies. Breakthrough T1D successfully accomplished this for AID systems—and we are confident that following a similar roadmap for cell therapies will get us to the finish line, faster.
The journey of AID systems
Learn more about the critical role of Breakthrough T1D in driving AID systems forward, recounted by Breakthrough T1D volunteer Doug Lowenstein.
Curative therapies for T1D are in reach. This roadmap, in conjunction with our Project ACT initiative, is key to bringing beta cell replacement therapies to every person with T1D who wants them.
To make this a reality, everyone needs to work together. As stated in the publication, “This requires a comprehensive strategy and a coordinated collaboration across stakeholders in every field relevant to islet cell replacement.” This roadmap is a guide for moving toward our common goal of a cure for T1D as soon as possible.
Breakthrough T1D will continue to lead the way until the T1D community can choose the beta cell replacement therapy that works best for them, regardless of blood glucose management or hypoglycemia status. Everyone deserves the chance to benefit.

