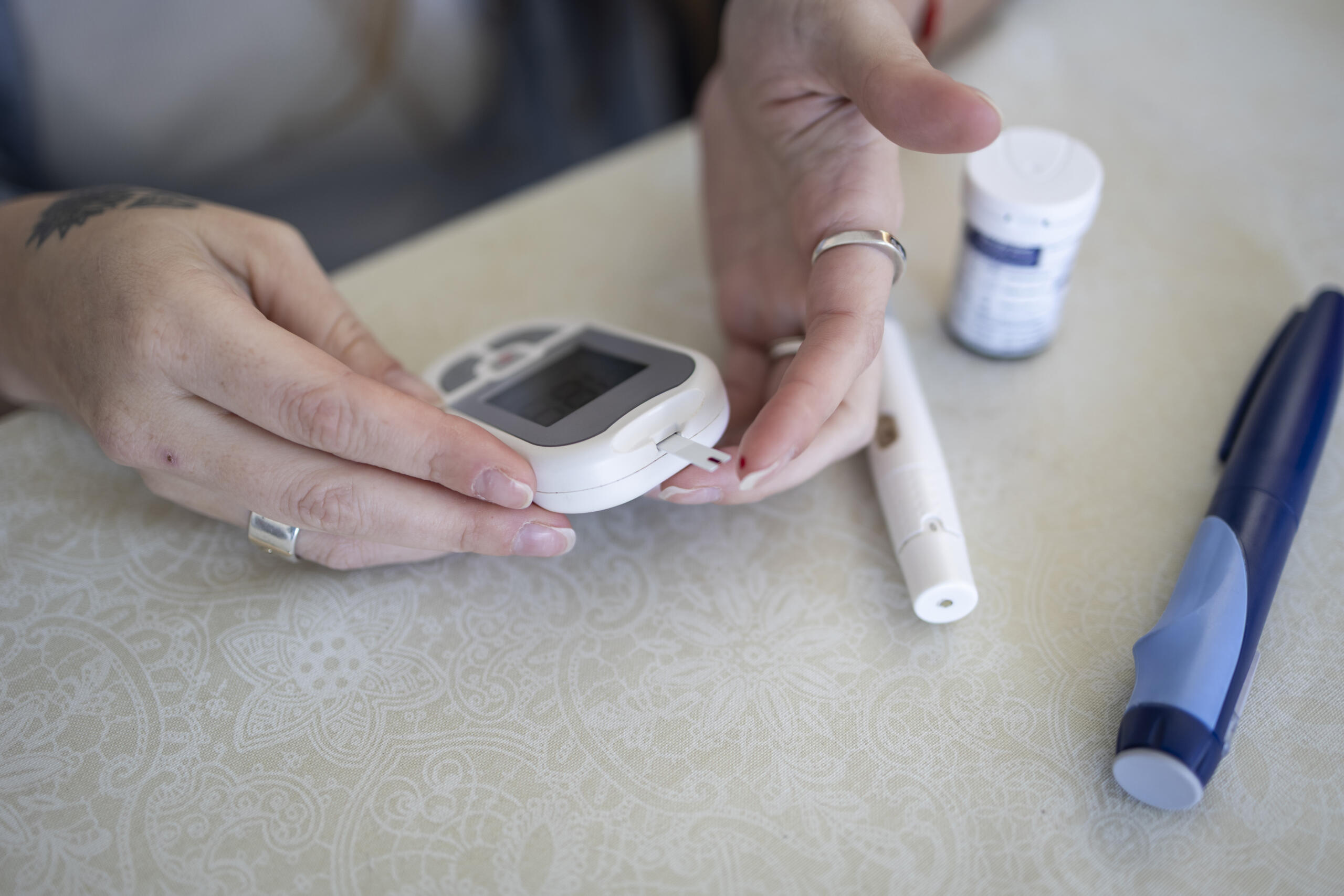
Navigating issues with your continuous glucose monitor (CGM)
Breakthrough T1D created a guide for sensor issues, from what to do if symptoms and CGM reading do not match to how to get a replacement.

Q&A with San Diego Padres Pitcher Mason Miller
Breakthrough T1D sat down with Mason Miller to learn more about his journey with type 1 diabetes and the impact it has on him as an athlete.

Hot off the press: Continuous ketone monitoring guidelines are here
What’s happening? Continuous ketone monitoring (CKM) is on its way. A new paper in The Lancet Diabetes & Endocrinology, titled “International expert recommendations on the application and utility of continuous ketone monitoring for people with diabetes,” was published by Breakthrough T1D and expert collaborators, spearheaded by our Chief Medical Officer, International, Thomas Danne, M.D., Ph.D. […]

Smart devices, angry skin: The underside of diabetes tech
Skin reactions from adhesives on insulin pumps and CGMs are common. Learn why they occur and how to reduce irritation.
Demystifying the pipeline of T1D therapies and devices: Turning ideas into reality
What is the pipeline? Every new medical device, therapy, treatment, and drug—including those for type 1 diabetes (T1D)—goes through the drug development pipeline. Getting a new therapy or device from the earliest stages of research eventually into the hands of people with T1D is a complicated process. Science takes time (from years to decades!), money […]

Special Diabetes Program yields $50+ billion in Federal healthcare savings
Analysis compiled by Avalere Health and supported by Breakthrough T1D (formerly JDRF) finds that research funded by the Special Diabetes Program (SDP) has yielded more than $50 billion in federal healthcare savings. Created by Congress in 1997 and administered by the National Institutes of Health (NIH), the SDP provides federal funding to support research to prevent, treat, and […]

Marshalls and Breakthrough T1D: Local community, global impact
As Breakthrough T1D’s largest corporate partner, Marshalls has raised more than $47 million to-date to improve and change life with type 1 diabetes.

How do school cell phone bans impact students with type 1 diabetes?
For students with type 1 diabetes who use technology to manage the condition, school cell phone bans can present challenges.

Can continuous glucose monitors benefit people without type 1 diabetes?
Continuous glucose monitors have been transformative for daily type 1 diabetes management. Can they benefit people without diabetes?

ADA 2025 Recap: Improving Lives
Improving Lives Breakthrough T1D’s Improving Lives program focuses on devices, insulins, adjunctive therapies, treatments for complications, and psychosocial interventions to improve the health and quality of life of people living with T1D. Adjunctive therapies and complications There was significant focus on GLP-1 receptor agonists (GLP-1RAs) and SGLT inhibitors (SGLTi) in reducing long-term complications and improving […]