
Dexcom G7 Continuous Glucose Monitor Cleared by the FDA
Dexcom G7® Continuous Glucose Monitor system was cleared by the FDA, giving people with diabetes more options for managing their blood-sugar levels.

FDA Approves Tzield™—A Watershed Moment for the T1D Community
Breakthrough T1D celebrates the FDA approval of Tzield, the first disease-modifying therapy to delay clinical T1D in people at risk of developing the disease.
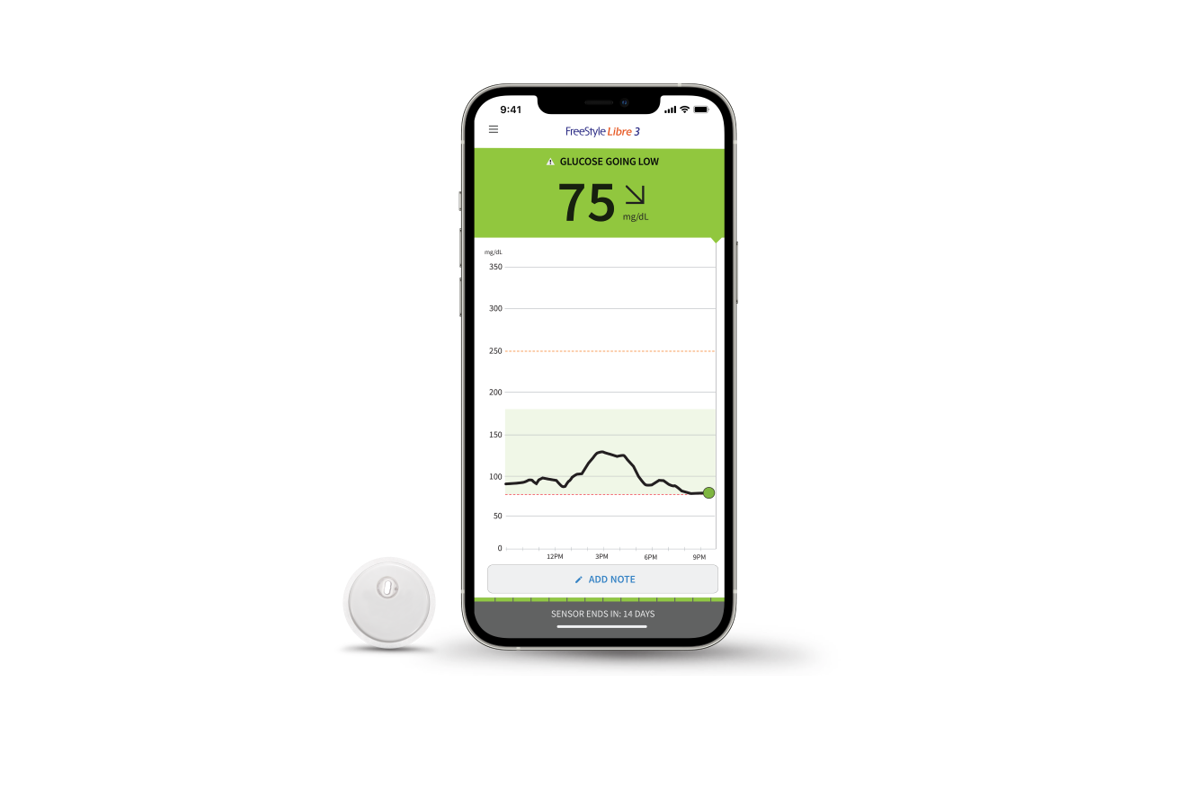
FDA Approved! Abbott FreeStyle Libre® 3 Continuous Glucose Monitor, Ages 4+
The FDA cleared the FreeStyle Libre 3 continuous glucose monitor (CGM) for children and adults ages 4 and up with diabetes