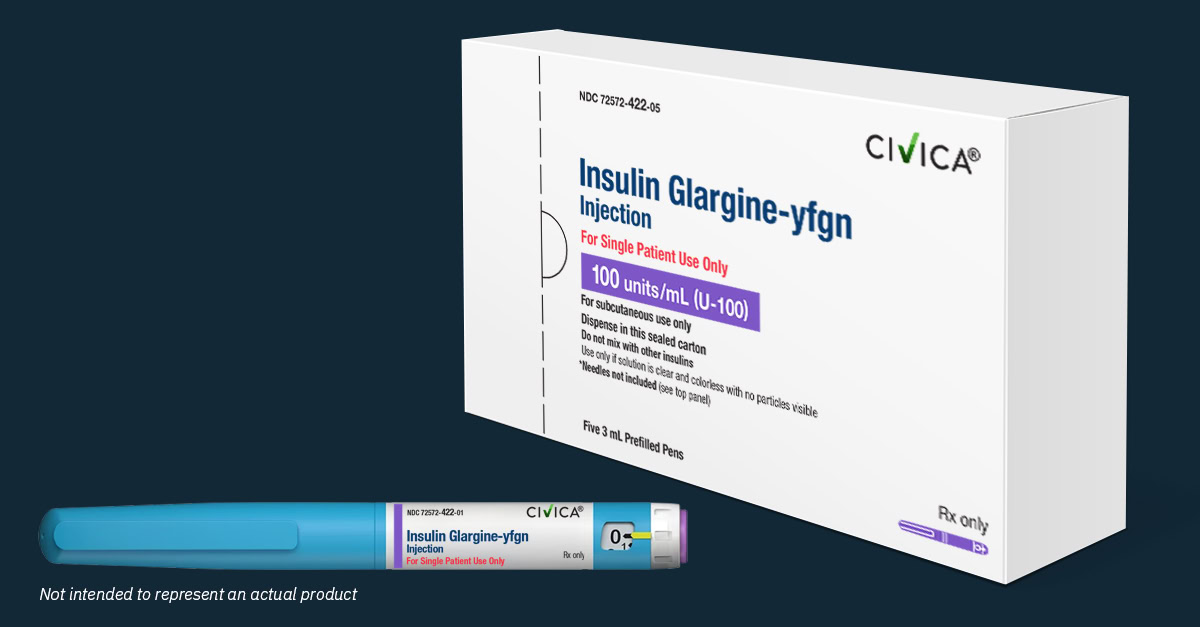
Civica’s affordable long-acting insulin now available!
Nearly 3 years after Breakthrough T1D joined forces with Civica, a non-profit pharmaceutical company, its first insulin is now available for purchase at pharmacies across the country. Check out our story from October that highlights the years of partnership between Civica and Breakthrough T1D that made today a reality. We worked with our partners at […]
Demystifying the pipeline of T1D therapies and devices: Turning ideas into reality
What is the pipeline? Every new medical device, therapy, treatment, and drug—including those for type 1 diabetes (T1D)—goes through the drug development pipeline. Getting a new therapy or device from the earliest stages of research eventually into the hands of people with T1D is a complicated process. Science takes time (from years to decades!), money […]
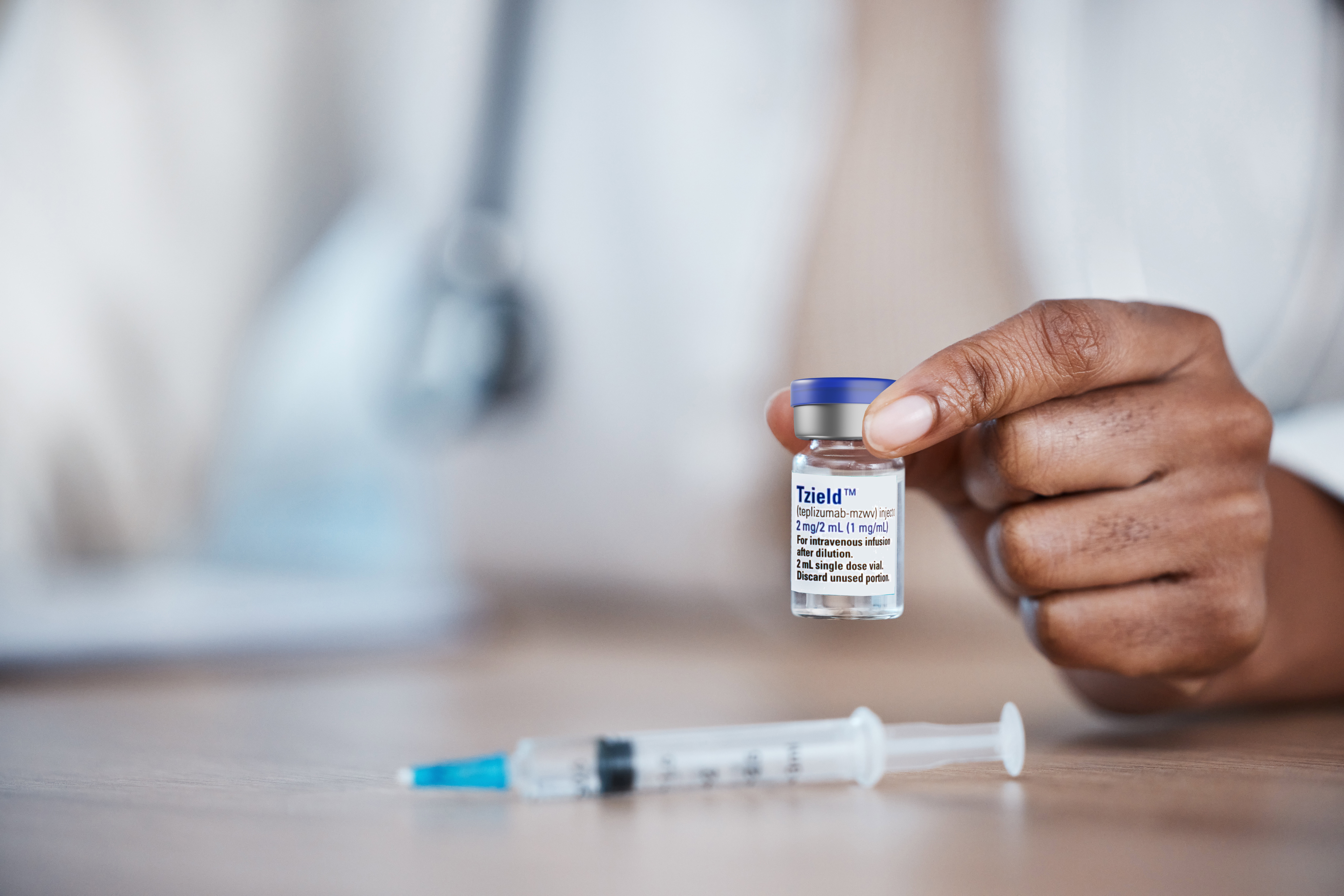
Tzield receives voucher for expedited review in stage 3 T1D
This past week, the FDA and Sanofi, the maker of Tzield, made an important announcement. Tzield, the first disease-modifying therapy approved for delaying onset of stage 3 type 1 diabetes (T1D) in people with stage 2 T1D, has been accepted into the FDA Commissioner’s National Priority Voucher (CNPV) program for people with stage 3 T1D. […]
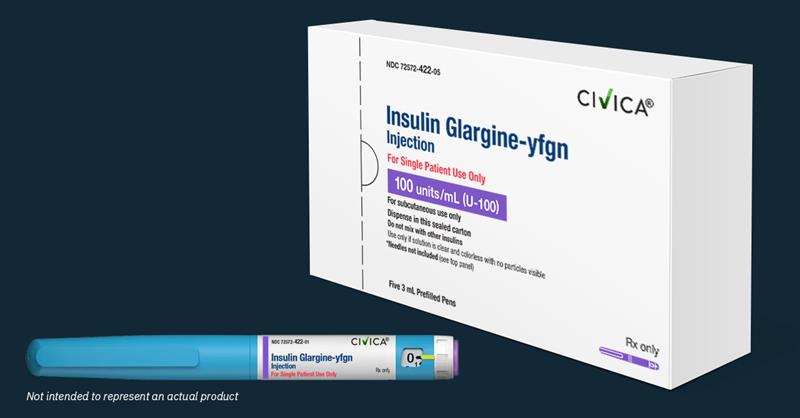
Civica long-acting insulin available on January 1, 2026
Nearly 3 years ago, Breakthrough T1D joined forces with Civica Rx, a non-profit pharmaceutical company, to manufacture and distribute low-cost insulins. Starting on January 1, 2026, the first of those insulins, insulin glargine-yfgn (a long-acting insulin interchangeable with Lantus®), will be available for purchase. 5 Pens, No More Than $55 – for anyone While we […]

New Breakthrough T1D publications highlight unmet needs and demographics of the T1D population
Breakthrough T1D recently published two peer-reviewed journal articles. One detailed burdensome unmet needs in the T1D community and identified key steps we can take to meet these needs. The other used real-world data to better understand American T1D demographics and predict changes in the next decade.

Challenges and solutions: The present and future of cell therapies for T1D
Breakthrough T1D is funding research that supports manufactured islet cell source, survival, and protection and is also driving initiatives to unlock access to manufactured islet therapies for people with T1D.

Breakthrough T1D 2025 Government Day: 4 amazing days, 485 Congressional meetings, 370 sore feet
Breakthrough T1D headed to Washington D.C. where advocates held nearly 500 meetings with Members of Congress on behalf of the T1D community.

2024 greatest T1D hits: A glance at this year’s achievements
While we look back on 2024, we can reflect upon the incredible progress we’ve made in advancing breakthroughs toward cures and improving everyday life with T1D. This wouldn’t have been possible without each and every one of you and your continued support of our mission as we drive toward cures for T1D. Here are the […]

New ICD-10 Codes Will Help the T1D Community
On October 1st, the T1D community got a big win: the Centers for Medicare and Medicaid Servies (CMS) introduced a unique ICD-10 code for stage 2 type 1 diabetes (T1D). This addresses a significant gap in clinical care. Prior to this change, clinicians had limited options for coding stage 2 T1D, which resulted in inaccurate […]

Advocating for progress and access
With the support of our grassroots advocates, our work helps advance treatments, influence policy, and improve access to care for those worldwide who need it.