
FDA Approves 7-day Infusion Set
Medtronic’s Extended Wear Infusion Set (EWIS) is the first infusion set approved for 7 days, a significantly longer duration; Currently, no other infusion set is approved for more than 3 days.

SFC Fluidics, a Breakthrough T1D Partner, Receives Breakthrough Device Designation
SFC Fluidics, a Breakthrough T1D partner, received a breakthrough device designation for its interoperable insulin delivery pod. How is it different? Find out here.

FDA Approves an Artificial Pancreas System for Children Aged 2-6 Years
The Food and Drug Administration (FDA) approved the Medtronic MiniMed 770G artificial pancreas system for use by children aged 2 to 6 with type 1 diabetes (T1D). It is the first marketed device that can automatically adjust insulin delivery based on continuous glucose monitoring (CGM) values for children aged 2-6 years.
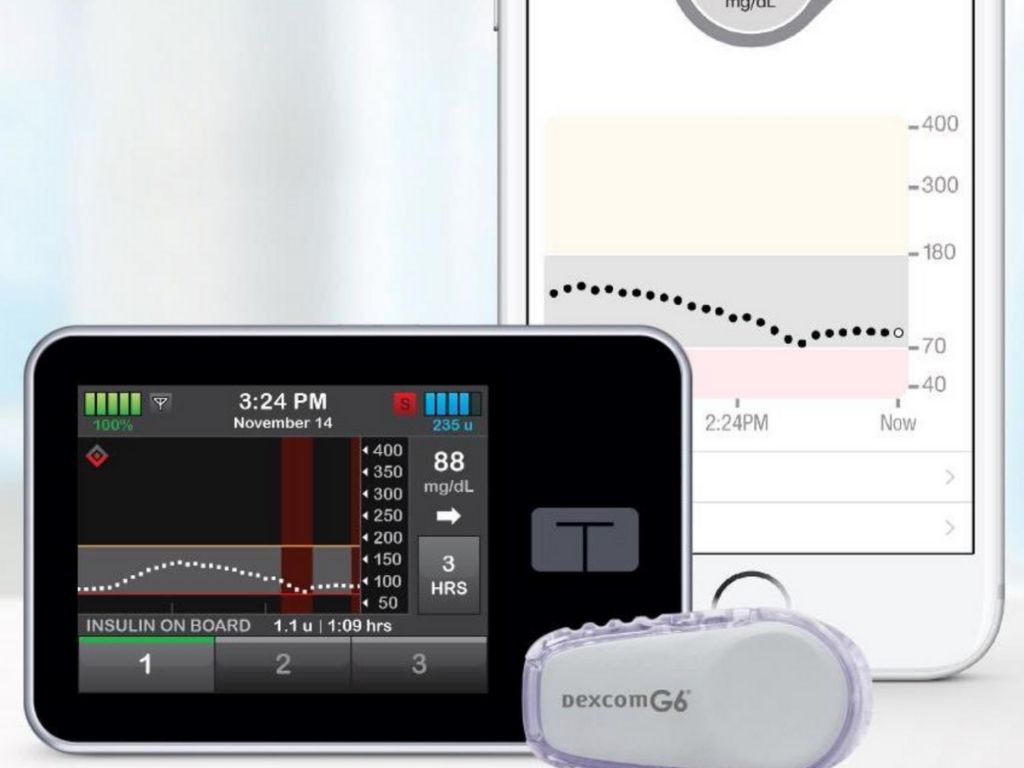
Tandem Control-IQ Improves T1D Management in Children Ages 6 and Up
A clinical trial at four pediatric diabetes centers in the United States has found that a new artificial pancreas system—which automatically monitors and regulates blood-sugar levels—is safe and effective in children as young as age six with type 1 diabetes (T1D): Tandem’s t:slim X2™ insulin pump with Control-IQ™ technology. The algorithm in the Control-IQ technology […]
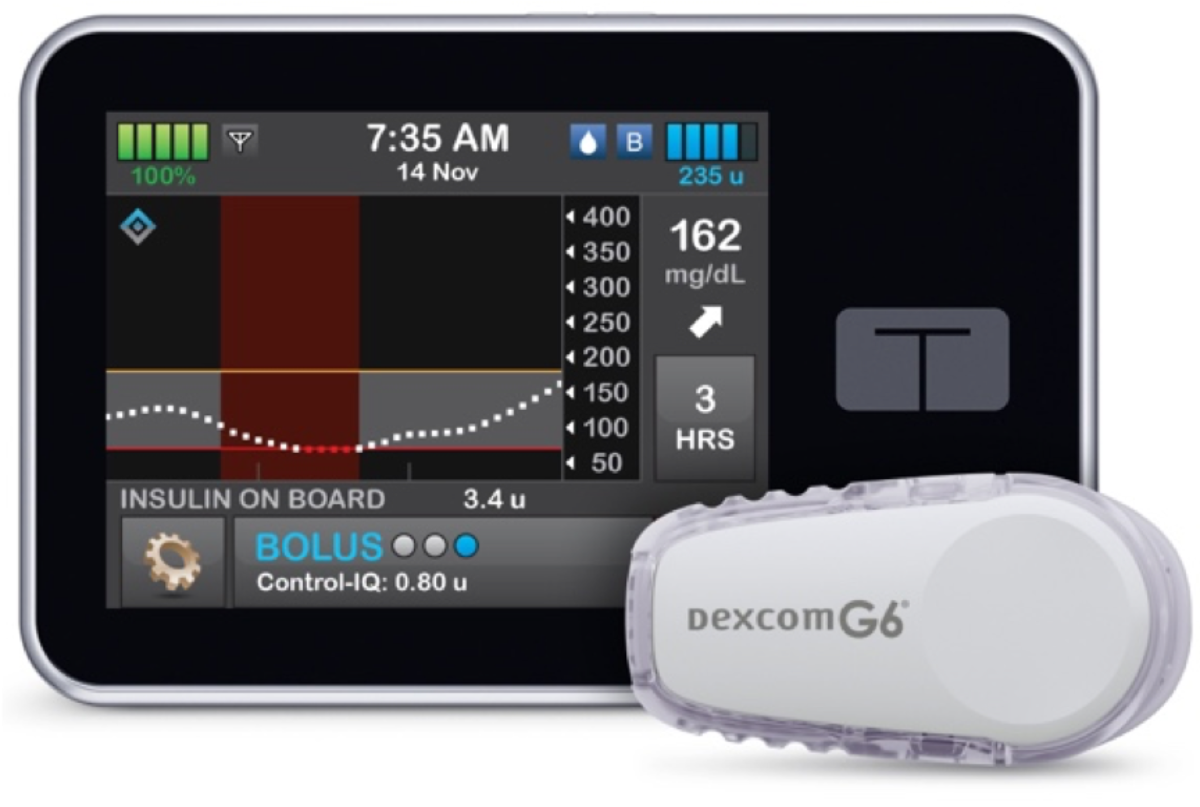
Tandem Control-IQ—Now Authorized For Children
Tandem Diabetes announced FDA clearance of an expanded pediatric indication for the Tandem t:slim X2™ insulin pump with Control-IQ™ technology in children ages six and older.

Breakthrough T1D and Gore Invest in Development of Implantable Insulin Delivery System
PhysioLogic Devices, a Breakthrough T1D-funded company that is dedicated to transforming the treatment of type 1 diabetes (T1D), wants to develop the ThinPump™—a fully implantable automated insulin delivery system—in the next four years. We think it’s not only possible, but doable. PhysioLogic has two things going for it: Gore is well known for developing GORE-TEX […]