
Powering research breakthroughs
We invest in the most promising research to turn ideas into life-changing therapies and lead the way to cures.
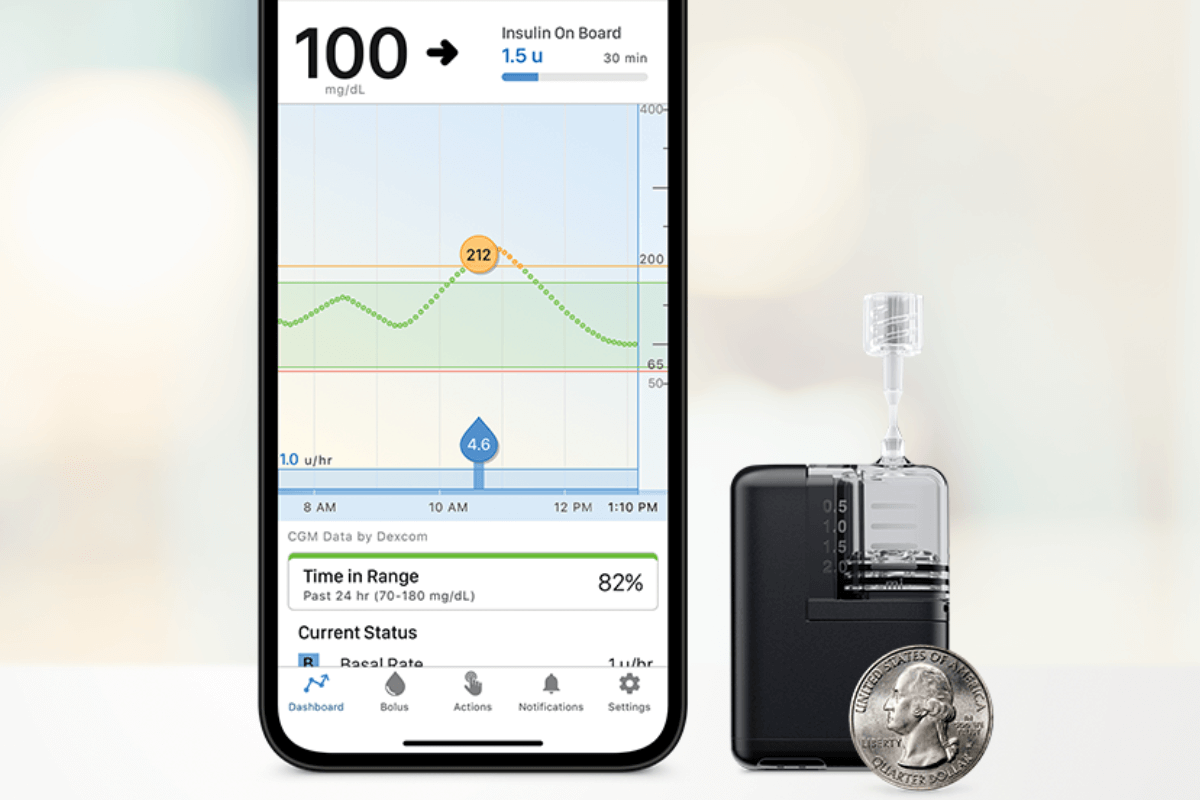
The Smallest Artificial Pancreas System Receives FDA Clearance
Tandem Mobi—a miniature-sized insulin pump, for use with Tandem’s Control-IQ™ technology and a compatible CGM—received FDA clearance.

Top Type 1 Diabetes Advances of Fiscal Year 2023
Breakthrough T1D highlights the top type 1 diabetes advances we've seen during fiscal year 2023, including cures and life-improving therapies.
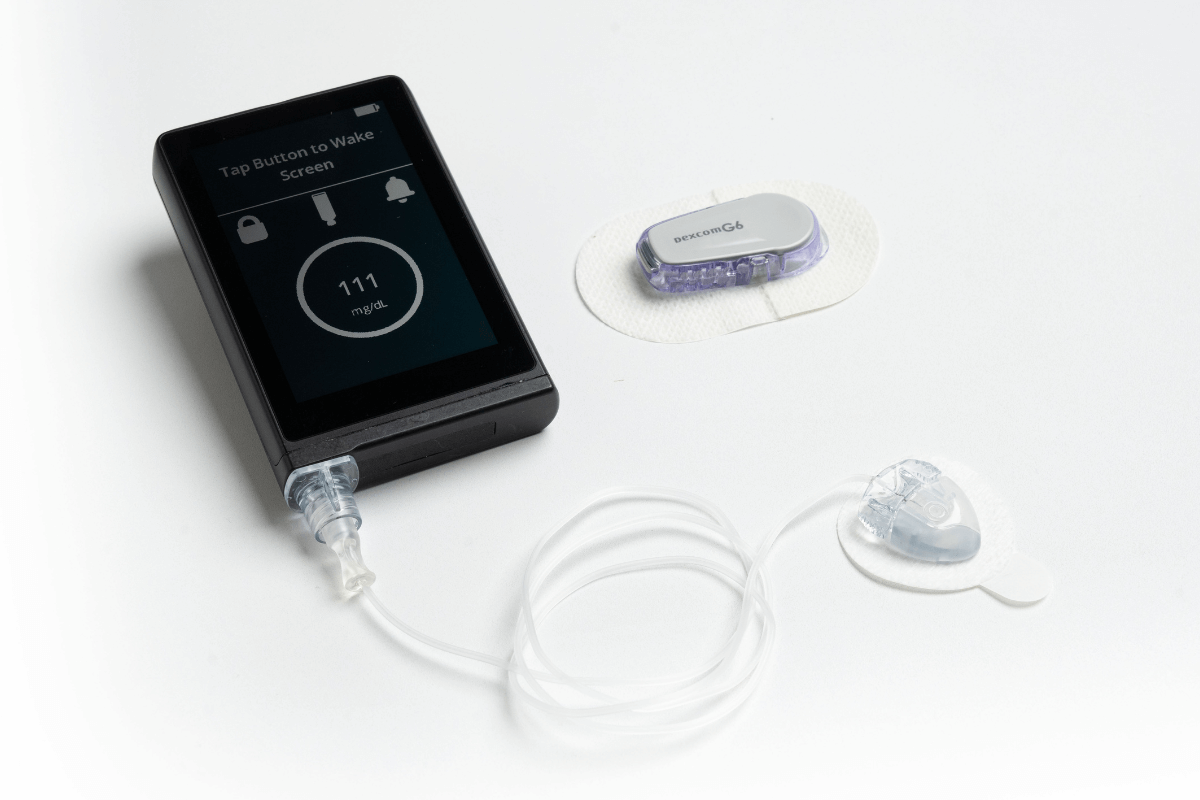
FDA Clears a New Artificial Pancreas System
The FDA cleared the iLet Insulin-Only Bionic Pancreas System for people 6 years of age and older with type 1 diabetes.
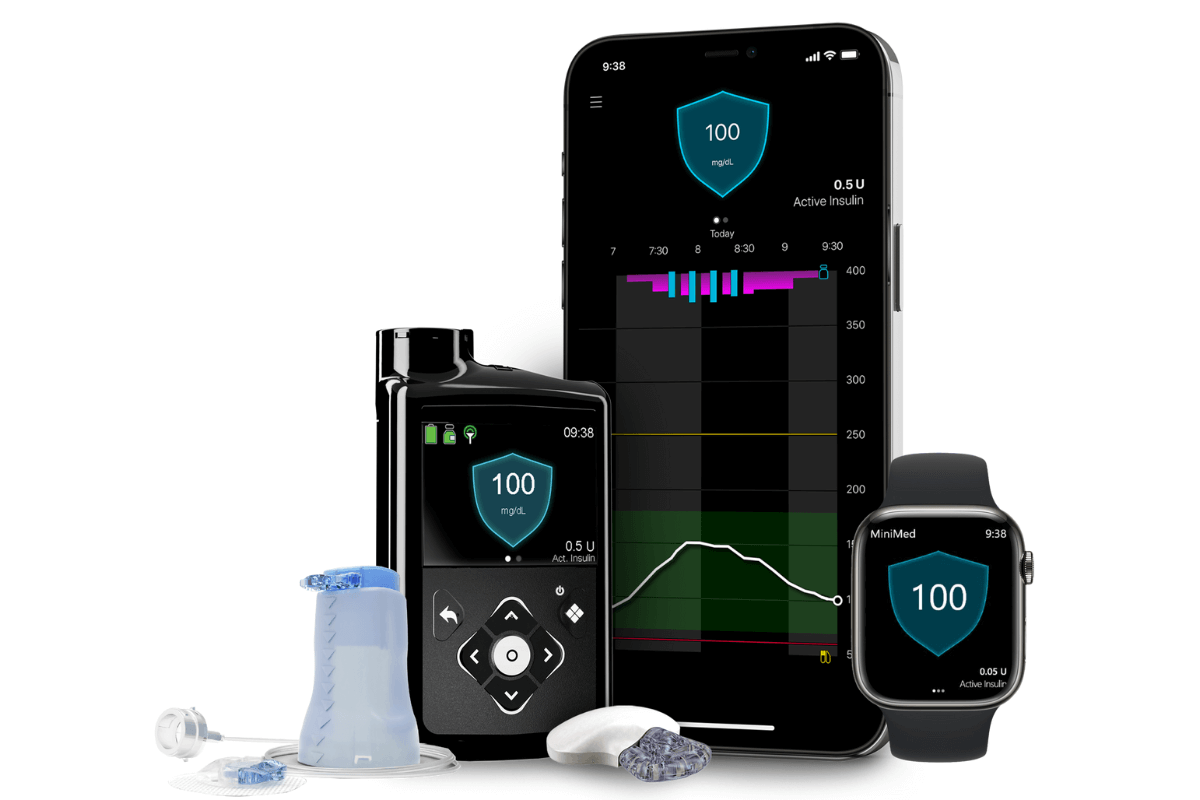
FDA Approves Medtronic 780G Artificial Pancreas System
FDA approved the Medtronic 780G artificial pancreas system, providing automatic adjustments and corrections to blood-sugar levels.

A Bittersweet Goodbye for Breakthrough T1D Champion Cynthia Rice
JDRF’s Chief Mission Strategy Officer will leave behind quite a legacy once she steps down from her role at Breakthrough T1D at the end of March 2023.

FDA Authorizes Tidepool Loop, an Automated Insulin Dosing App
The FDA authorized the Tidepool Loop, an algorithm that could be used to work with commercially available insulin pumps and continuous glucose monitors.
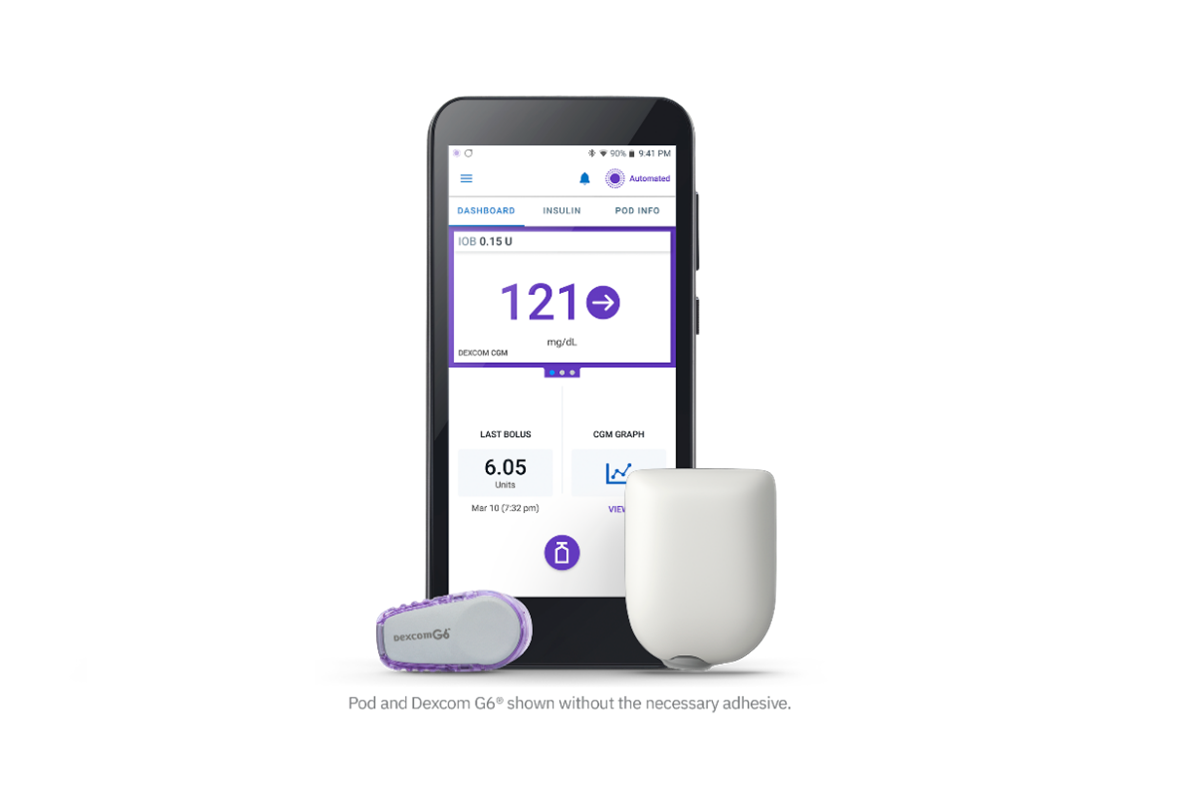
FDA Authorizes Omnipod 5 for Ages 2+ in Children with Type 1 Diabetes
The FDA authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system, for children 2+, using an algorithm that began by Breakthrough T1D funding.

Tandem Diabetes Makes Managing T1D More Convenient
Tandem announced the FDA clearance of bolus insulin dosing from their t:connect® mobile app. Soon, individuals with the Tandem t:slim X2™ pump will be able to bolus remotely from either their iOS or Android smartphone.

FDA Authorizes a Fourth Artificial Pancreas System
The FDA authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system for people 6 and older, using an algorithm that began by Breakthrough T1D funding.