
FDA Breakthrough Therapy Designation for Teplizumab—Based on the First Study to Delay the Onset of T1D for 2+ Years
The U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to teplizumab, an anti-CD3 monoclonal antibody, for the prevention…

FDA Approves Baqsimi: The First Low Blood Sugar Treatment WITHOUT an Injection
Type 1 diabetes (T1D) can bring the fear of developing low blood sugar, called hypoglycemia, which can arise if someone…
Enhanced Local Immune Suppression: Hope for Beta Cell Replacement Therapies
Medical devices such as pacemakers and stents that can be implanted in a human body have helped many people lead…

Breakthrough T1D Top Advances Over the Past Year
Thanks to the efforts of our donors and volunteers, we have made a series of top advances in 2019 type…

At ADA, Breakthrough T1D-Funded Research Takes Center Stage
Breakthrough T1D was there when the top type 1 diabetes (T1D) experts from all over the world convened this month…

ADA Scientific Sessions Recap—Day #5: Marlon Pragnell, Ph.D.
The ADA’s Scientific Sessions is completed! Here, with the post-day recap, is Marlon Pragnell, Ph.D., head of the complications program…

Two T1D Technology Leaders Partnering to Drive Innovation and Choice
Two leading innovators in the type 1 diabetes (T1D) space have announced a collaborative partnership aimed at enabling those with…

ADA Scientific Sessions Recap—Day #4: Simi Ahmed, Ph.D.
The American Diabetes Association’s Scientific Sessions is almost coming to a close! Here is Simi Ahmed, Ph.D., head of the…

ADA Scientific Sessions Recap—T1D Prevention Research: Jessica Dunne, Ph.D.
There was some really exciting T1D prevention research in day #3, and also screening for type 1 diabetes risk. Here…
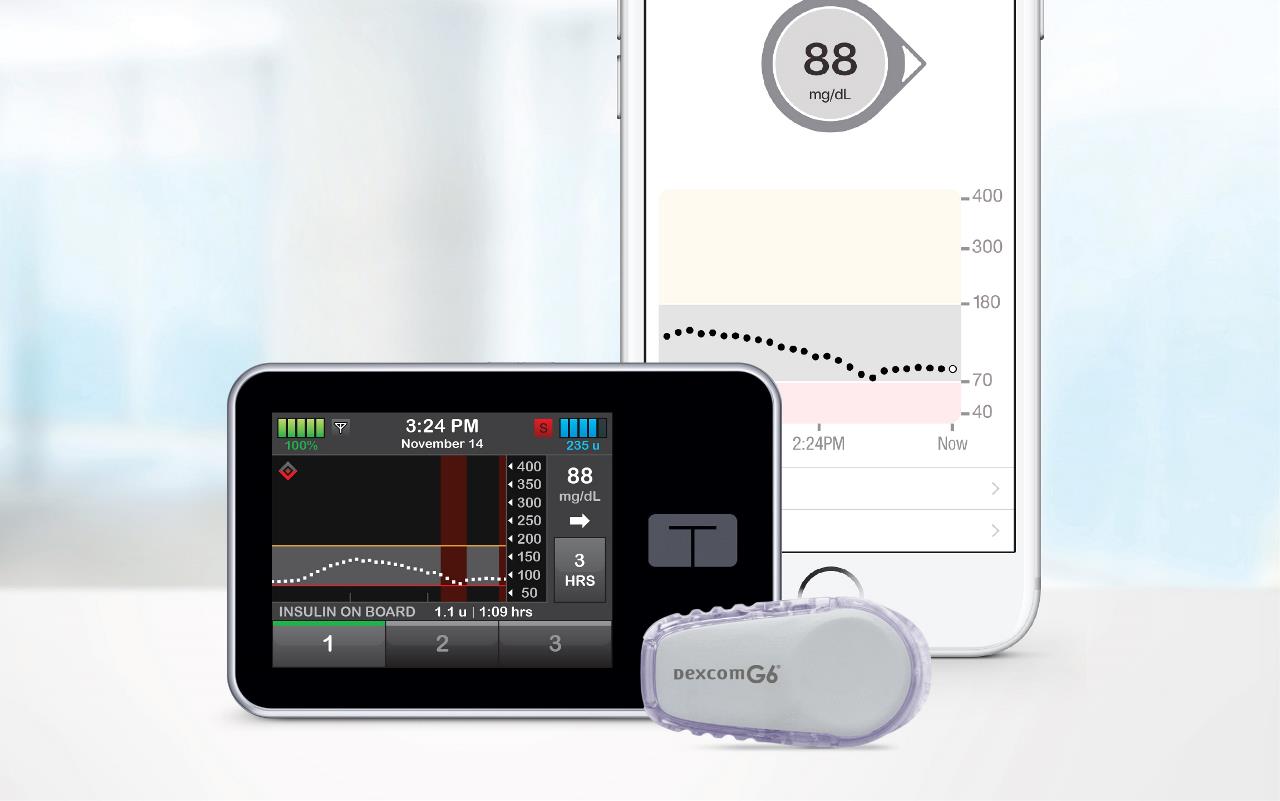
A New AP System by Tandem Shows Key Time-in-Range Benefits
A new artificial pancreas system that both doses and takes away insulin is showing critical benefits in helping those with…