
Vertex Pharmaceuticals Investigating Cell Therapy for Type 1 Diabetes
Vertex is currently enrolling for a clinical trial evaluating their stem cell derived islet cell infusion therapy, VX-880.

Back to School with Diabetes Amidst the COVID-19 Variants
Kids with diabetes need to get back to school, but how can we best keep them safe? Experts weigh in.

Breakthrough T1D & NIH Workshop at the Intersection of Immunology and Bioengineering
Breakthrough T1D and NIH bring together top scientists and engineers to strategize on how to allow implanted beta cells to…

Kid-Approved Back-to-School T1D-Friendly Recipes
Tasty, easy-to-make diabetes-friendly treats for school lunches, after school snacks, and school night dinners.

Abbott FreeStyle Libre 2 iOS App Receives FDA Approval
On August 2, 2021, Abbott announced the FreeStyle Libre 2 iOS app received approval from the FDA for adults and…

FDA Approves Semglee® as the First Interchangeable Biosimilar Insulin Product
The U.S. Food and Drug Administration (FDA) approved Semglee® (insulin glargine-yfgn) as the first ever interchangeable biosimilar insulin product. Semglee®…

Two Studies Shed Light on Diabetic Kidney Disease
Two recent publications shed light on which people with type 1 diabetes will develop end-stage kidney disease, a complication of…
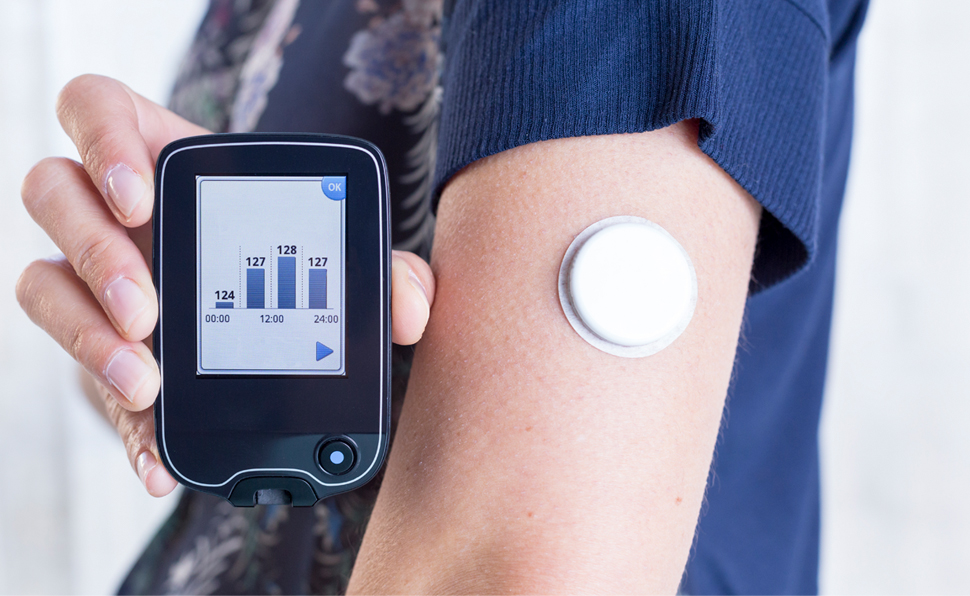
On Medicare? Accessing CGMs Just Got Easier
A recent update to continuous glucose monitor (CGM) coverage requirements will make it easier for people covered by Medicare to…

Eli Lilly Acquires Breakthrough T1D T1D Fund Portfolio Company Protomer Technologies
Breakthrough T1D is excited to announce that the T1D Fund portfolio company Protomer Technologies, who is developing "smart" insulin, has been acquired by Eli…

Teplizumab (the Therapy that Delays T1D By Nearly 3 Years) Isn’t Approved…Yet
The FDA issued a letter to Provention Bio for the use of teplizumab to delay T1D in at-risk individuals, meaning…