Breakthrough T1D Connection Tool
Disease Modifying Therapies (DMTs) are therapies that work on reserving and restoring the pancreas’ ability to make its own insulin. This restores the capabilities it had before autoimmune Type 1 Diabetes (T1D) occurred. In a healthy pancreas, your beta cells are the cells that produce the hormone insulin. Insulin is what allows glucose to enter cells in your body to be used as energy for cell function. In T1D, your immune system sees insulin-producing beta cells as cells that it should attack, and then it destroys these beta cells. As more and more of these beta cells are destroyed, the glucose level increases in your blood. When the glucose level gets too high, this is when you will need injected insulin to survive. This is when T1D is diagnosed in the clinical setting.
In T1D, Breakthrough T1D is working on DMTs to either prevent, halt, or reverse the loss of those insulin-producing beta cells. This will change the course of the disease. The new therapies will need to take into account that there are a number of different stages involved when T1D progresses. This will mean that therapies may be targeted toward the immune cells that are involved in the attack, or therapies for the beta cells to help them stay healthy and protect their survival. T1D does not start the day you are diagnosed, as by then it has already been on a path of an autoimmune attack, often for many years prior to diagnosis. When you have yet to experience the clinical symptoms of T1D, you will be unaware of the formation of your first autoantibodies involved in T1D. Your glucose level will experience undetectable highs and lows and it becomes more dysregulated; this is stage 1. Specifically, stage 1 is when you have normal blood sugar and autoantibodies circulating in your blood, meaning two or more of these autoantibodies. The only way to detect stage one is to test for these autoantibodies. This can currently be done either through TrialNet, which is for those with relatives with T1D, or now by using a T1D detect kit which can be ordered and is available for the general public. Most who develop T1D, about 90%, do not know of any family connection to T1D. When a person goes from stage 1 (normal blood glucose) to stage 2, their insulin-producing beta cells start to function poorly, as the cells are stressed or dying. The only way to know if you have progressed from stage 1 to stage 2 to go to your doctor’s office and have your doctor do specific metabolic testing, now only in a clinical trial setting. This metabolic change occurs when a significant number of insulin-producing cells have been destroyed by the immune system. As beta cells are destroyed, insulin injections will be started in order to survive. The cure strategy for Breakthrough T1D with DMTs has two main strategies: the first is to turn off the autoimmune attack against the beta cells. We need to stop this attack, as it will continue to happen until there is an intervention that stops this process. The second approach to the DMT is to help sustain the survival of existing beta cells and encourage your own body to create new beta cells. Breakthrough T1D is accelerating both types of research for new therapies. Breakthrough T1D does not stop there. As these therapies are closer to FDA regulatory approval, Breakthrough T1D is very active in making sure these promising therapies get to the patient. More on that to come in this article. One thing that is apparent is that we need to do more screening, as we cannot prevent someone from developing T1D unless we know they have begun to form autoantibodies that are connected to T1D. If this is not known until someone is clinically diagnosed with T1D, there is a 60% higher chance of being diagnosed in diabetic ketoacidosis. This can be a very dangerous situation and will involve hospitalization, and can cause damage to other organs. Screening can greatly reduce risk of more serious events at diagnosis by being able to monitor the disease’s progress before it becomes a new case of clinically diagnosed T1D. The most important reason to pick up the formation of these early autoantibodies is that there are clinical trials that are currently running to test treatments that Breakthrough T1D sees as having the potential to cure and prevent T1D. These cures being available are dependent on people participating in clinical trials to accelerate development of these therapies. This includes those who are either at risk for developing the disease, or recently diagnosed with T1D. Most current screening programs are for the pediatric community. Of those that are newly diagnosed with T1D currently, it is known that around 50% are in the adult community. Breakthrough T1D is working to help change health policy to include screening for both communities and have it become part of screening in our healthcare systems. A general population screening will better capture everyone who is at risk, not just those with a known family connection. The current screening programs such as TrialNet have also been able to benefit families, as they provide support while a participant travels from being in an at-risk group to knowing they have formed autoantibodies for T1D. For those that have formed these autoantibodies, clinical trials have been able to delay the onset of clinical T1D for at least 3 years and counting! These studies continue to support families as they go through what might be a difficult diagnosis. There is education, personal, and medical support in a study. You need to be monitored and working with a doctor through your risk diagnosis. T1D also occurs in all demographics and we hope to have a diverse population represented in screening programs and clinical trials in order to help stop T1D in all groups.
Breakthrough T1D is working to educate people about T1D and its risk. This needs to be targeted at the general population also, which means teaching healthcare providers as to why everyone should be screened. The best tool currently for the general population is the T1Detect Kit. This test costs about $55. If you cannot afford the cost, there is an assistance fund that can help to bring the cost down to $10. When it is sent to your home, it will give you instructions on how to send back a simple blood test that is provided. The spot of blood on a card will be able to detect the major autoantibodies that can detect whether you have autoimmunity for T1D. If there are autoantibodies present, they will also be confirmed by additional test. Those with T1D can also teach family and friends about the importance of knowing this T1D risk.
The graphic below shows current screenings that are occurring globally for those with a connection to someone with T1D. The second graphic shows the screenings that are available globally to the general population.
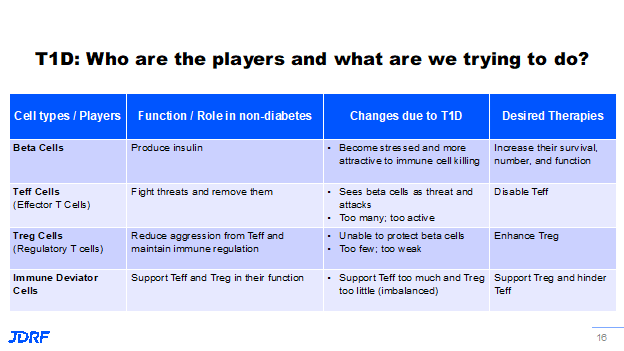
Let’s delve into some of the science in DMTs. In other words, how are we going to turn off the autoimmune cell attack and how can we improve the survival of or regrow beta cells? Breakthrough T1D is looking for therapies that can improve beta cell survival and these therapies have been shown to improve the survival of beta cells and are currently in clinical trials now. Verapamil, a blood pressure drug, is one example. This drug is in multiple trials around the globe and it is also being tested in those that have recently been diagnosed with T1D to see if it will also help with beta cell survival after diagnosis. Another cell type in your body that is being looked at is the T effector (Teff) cells. The Teff cell’s role is to fight off infection from bacteria and viruses then remove them. However, in T1D autoimmunity they instead get directed to the beta cells in your pancreas. For some reason there are too many of this type of cell circulating and their attention is turned to the wrong cell target, the beta cell. Breakthrough T1D is working to find therapies that can either disable these cells or reduce their numbers. One example is Teplizumab. It is being tested in multiple stages of T1D. It is currently awaiting FDA approval as a drug that can delay the onset of the clinical diagnosis of T1D in the at-risk community in stage 2. Another cell that is important to address is the T regulatory (Treg) cell. A Treg cell is considered a peacekeeper cell that helps regulate cells and provide a balance to the Teff cells. In T1D we know there are not enough of these cells and those that are present are not so great at their job. New therapies are being developed to enhance the work they do and to have more of them present. This is being done through antigen-specific therapies that are being tested. Novo Nordisk is currently running a trial called TOPPLE, where they are using a DNA vaccine to produce antigen-specific Treg cells for insulin. These will be peacekeeper cells that are meant to instruct cells that want to attack the insulin-producing beta cells to calm down and not attack. The third type of cell being worked on is the immune deviator cell. These are cells as part of the immune system that support both Treg and Teff cells. In T1D, they tend to not be good at their job, either, and end up supporting the T cells that attack the beta cells. They are not efficient at supporting the Treg cells. Breakthrough T1D is working to have the immune deviator cells create a bias toward supporting the peacekeeping Treg cells. See the helpful graphic below to help with remembering the roles of these cells for understanding future therapies.
One of the exciting trials is the BANDIT trial that launched in Australia in December of last year. The BANDIT trial is using a class of drug called a JAK inhibitor (JAKi). A JAKi is a type of immunosuppressive drug that is currently useful in multiple autoimmune diseases. It is a drug that is already approved for other autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis. Breakthrough T1D has funded research with an academic investigator in Australia named Dr. Helen Thomas. She is working to prove that this class of drugs, JAKis, could be effective in T1D. Breakthrough T1D was instrumental in encouraging Eli Lily, the maker of the drug baricitinib, to provide the drug for this clinical trial. This will be the first ever trial using a JAKi in this new concept to see if it can keep beta cells alive and keep the immune system from attacking. The results of this study are targeted to be available in 2023. We are looking to see similar trials here in the US.
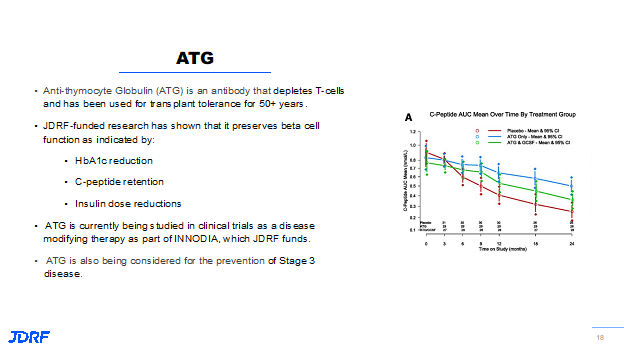
Another drug that is being looked at is ATG, anti-thymocyte globulin. It is a type of antibody that can specifically remove T cells from circulation. Remember the Teff cells (bad guys) and the Treg cells (good guys). This ATG has been found to be capable of removing the Teff cells from circulation. Breakthrough T1D has been funding research around ATG for a number of years. We know that if you treat people after diagnosis with T1D with ATG, it can preserve beta cell function in producing insulin. It was shown to reduce the need for exogenous insulin. We can measure the ability to produce insulin by looking at the C peptide. Looking at this slide below, people in the red line are the people not taking the drug. They continued to decline over the course of the two years of study treatment. The people in blue who were on ATG had preserved their ability to produce insulin as observed by the C peptide level. ATG is now being studied in the INNODIA consortium in Europe, and is getting ready to be studied here in the US and with other clinical trial consortiums. It is being studied as a preventative as a way to delay the onset of T1D. The drug ATG is produced by Sanofi.
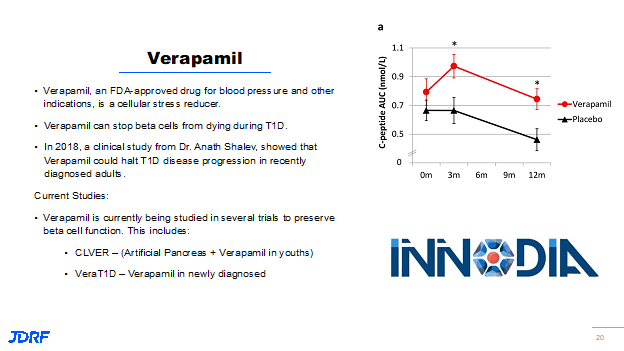
The next promising drug is Verapamil. Verapamil is a blood pressure drug that was shown by Dr. Anath Shalev, of the University of Alabama, to reduce the beta cell stress that occurs with T1D. The reason this is important is that when beta cells get stressed, they can’t function as they should and therefore can’t produce the needed insulin. As the beta cells continue to be stressed, they end up dying. Dr. Shalev has initially showed this drug could preserve beta cell function in animal models with T1D. Because of this finding, Breakthrough T1D worked with Dr. Shalev to do a clinical study that showed if this drug is given to people recently diagnosed with T1D, the beta cell function can not only be preserved, it can also be kept stable.
If you look at the slide for Verapamil below, the red line are the people who are treated with Verapamil. You can see from zero to three months they have a little bit of a boost in their ability to produce insulin. That then declines a little over the course of the next nine months. But people who are on placebo do not show that increase in insulin production as did those on Verapamil.
With this important finding, Verapamil is being used now in multiple clinical studies. In the US it is being used in the CLVer study, which is combining a pump device system such as an artificial pancreas with Verapamil in very young children to see if there is preservation of beta cell function after diagnosis. The Ver-A-T1D study in Europe is a larger study to repeat the findings found by Dr. Shalev and apply the findings to a wider population.
The above examples is a small subset of all the trials that are going on and being funded by Breakthrough T1D. Breakthrough T1D is working at all stages of the disease. We know from clinical trials that we are making progress. Along with the development of these new therapies, Breakthrough T1D has also been leading the charge in making sure these life changing therapies make it to people who need them. Breakthrough T1D works with investigators and with companies to convince them as to what the right path forward is and how working with critical consortia (scientific groups working on the same type of research) can help in studying intended populations accurately. Breakthrough T1D has the best team to navigate the regulatory pathway to approval. This is a huge part that will enable therapies to get FDA approved, accepted, and prescribed by clinicians to get a DMT to the patient.
Breakthrough T1D has a robust advocacy and policy department. This department is critical to our success in finding cures and therapies for those with T1D. This advocacy department for Breakthrough T1D has a home in Washington DC. The government relations side focuses on getting the continual strong Federal support, such as the Special Diabetes Program. This currently provides $150 million a year in research dollars for T1D. This is one of the most successful public-private partnerships and one that has garnered support from both parties since its inception. The advocacy department also engages and supports tens of thousands of Breakthrough T1D volunteers across the country that help to educate not only members of Congress, but also those that work in our regulatory institutions such as the FDA. This is one of the most interesting volunteer jobs around. The Breakthrough T1D regulatory group works to make sure there is a logical regulatory pathway for further research, cures and prevention. The policy group is responsible for the work to make sure that these healthcare therapies can be accessed by everyone. Let us go on a deeper dive into examples of what this may entail. In research, outcomes and endpoints are something that regulators will use as their focus when looking at clinical trials for new therapies. One example is a HbA1c. This is a common endpoint along with test such as C-peptide and other markers of autoimmunity. The use of C peptide is a marker for endogenous insulin production, what you are producing on your own. Looking at test markers can tell a researcher how interventions may modify the immune aspects of T1D. The policy department also works with industry and researchers to make sure additional and appropriate outcome measures are incorporated into the development of the study design. Clinical studies can also use measurements obtained from the use of a continuous glucose monitor (CGM). This can provide data like Time In Range that may tell a researcher more about what is occurring.
Breakthrough T1D advocacy has been backing the CGM and artificial pancreas systems from their inceptions to academia, industry, and the regulators. This team presented the value of having the CGM data and measurements that a patient can use 24/7. The numbers help with treatment decisions and provide information that can be used to avoid future complications. The CGM data helps to show the FDA, patients, and caregivers what is happening in real time with treatments. There is a large amount of CGM data that can now be accessed when a CGM is being used in a clinical study. These large datasets will be able to help answer physiological questions of what is happening in a study. This information will be helpful for researchers for analyzing the benefits of new therapies, drugs, and devices.
Here is a bit of history as to how Breakthrough T1D advocated for the CGM. A landmark trial called the DCCT trial showed that HbA1c was a surrogate for your risk of future complications. In the development of the CGM, this helped to establish metrics. This helped to show regulators how the numbers that could be seen on a CGM can correlate with how a patient will feel and function, while also helping to have a much healthier future. Breakthrough T1D is encouraging both academic and industry trials to collect these large datasets they can gather from the use of a CGM. As was noted in a recent Breakthrough T1D talk by Marjana Marinac, the FDA’s position on CGM was initially called exploratory; they were not necessarily encouraging sponsors to collect this data. They were not encouraging sponsors of trials to fund the use of the CGM at that time. At this time the FDA called this Exploratorium funds, since not a lot was known about the technology. Breakthrough T1D is now seeing a shift within the FDA center involved with drug approval encouraging and asking sponsors to collect CGM data in the drug development process. Breakthrough T1D sees this as a positive sign, and it shows that they are now looking at these large datasets that can be mined to validate research findings.
Breakthrough T1D policy also plays a critical role in bringing consistency to the metrics used in T1D, whether it be CGM data, C- peptide, or HbA1C. They looked at the different research studies and noted the inconsistency in how the various metrics where defined. They were able to bring the research community together to find consensus on clinically meaningful outcomes measures and then helping to created standardized definitions to be used. Breakthrough T1D has also worked with patients and caregivers to identify preferred terms of outcomes beyond what is used with regulators. This Breakthrough T1D study found that patients and their caregivers prioritized addressing the frequency and severity of hypoglycemic events in hypoglycemic events. There are many ways that Breakthrough T1D helps to improve concepts and standardization.
In 2014 Breakthrough T1D was really at the front of identifying the stages found in T1D. We knew that T1D started many years before the diagnosis and becoming insulin dependent. Through studies that had been done, it was known that once you had two or more autoantibodies you were at a significant risk to develop T1D. They were able to bring the community together, including the regulators, to establish and agree on the staging of T1D. The FDA was also invited into these discussions. The information on autoantibodies was information that the FDA had not seen in publication before, so this was helpful. Regulatory works closely with those research experts and industry so the T1D scientific community can have consistency in the use of surrogate markers, such as C peptide. This includes discussions on the best designs for clinical trials and what they should look like for T1D. Regulatory also works with FDA to give them information that may lead them to give certain research breakthrough therapy designation. The FDA has done that in T1D when clinical data supports that this research is addressing and showing significant findings. This helps to accelerate the review and development pathways for therapies that have this designation.
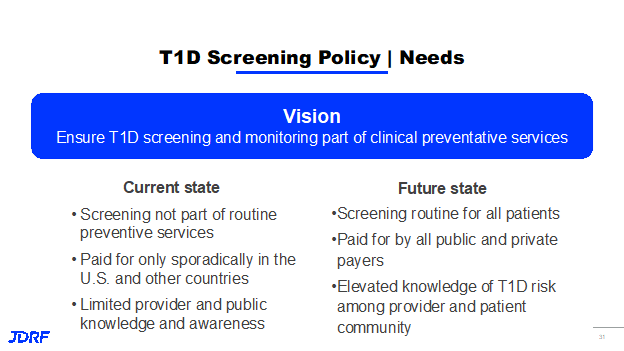
On the policy group, Breakthrough T1D is making sure that we have intervention and screening programs covered for T1D. We also need the support of physicians adopting these key programs that lead to better outcomes for people. Screening and monitoring programs should be part of clinical preventative services. Currently it is not part of routine prevention. In the US and Canada, its use is only occasionally reimbursed.
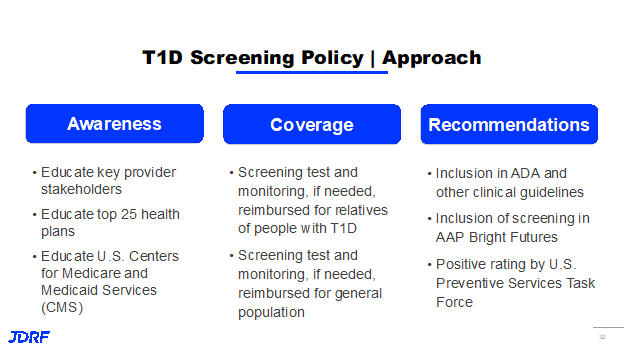
Part of this is because there is limited knowledge by the public and clinicians as to its benefits and what we want to see this program achieve. Breakthrough T1D would like to see it paid for by public and private payers, like other preventive screening programs are currently. Policy is working to achieve this goal by educating key provider stakeholders including the top 25 health plans. Part of this work is also educating CMS, US Centers for Medicare and Medicaid Services, to insure coverage. Breakthrough T1D is also seeking coverage for the general population and to have this screening included in the American Academy of Pediatrics Bright Futures Program. This step helps on the road to being added to preventive screenings.
Using the link below, there are not only more detailed discussions of the DMTs mentioned at the beginning of this article, but there are many more great examples of the work Breakthrough T1D advocacy, regulatory, and policy people are doing to get research to patients. If you are not already a Breakthrough T1D advocate, become an advocate. This is the easiest and most important Breakthrough T1D volunteer program you can be part of and it takes very little time for you as a volunteer to be effective. It is also the best resource to get information on what is happening with T1D Federal funding and therapies that need your input to help move Federal regulators to act. I have been active in advocacy for 20 years and the work that has been accomplished in this timeframe has been amazing.
This article is a synopsis of the Breakthrough T1D YouTube Research Talk on Disease Modifying Therapies from December 2021, here is the link to the full talk by Dr. Frank Martin, PhD, Senior Director Breakthrough T1D Research and Marjana Marinac PharmD, Senior Director, Breakthrough T1D Regulatory Director. There are many great research talks available on the Breakthrough T1D YouTube channel.
Most importantly, there are many clinical trials available covering many different therapies and stages of T1D. At the top of this article there are links to these trials. The Breakthrough T1D Connection tool allows you to input your specific information and gives you the trials that you may qualify to participate in.
Please feel free to reach out to me with any questions you have about the research that is currently going on, along with questions about clinical trials in our area. Even if it is to have a chance to talk through the concepts so you can understand them more and be able to share the promise we are now seeing for the future of T1D. I can be reached at debbieaevans1@gmail.com. I can also be reached at 612-810-1933 – please leave a message and I will call you back as soon as possible.