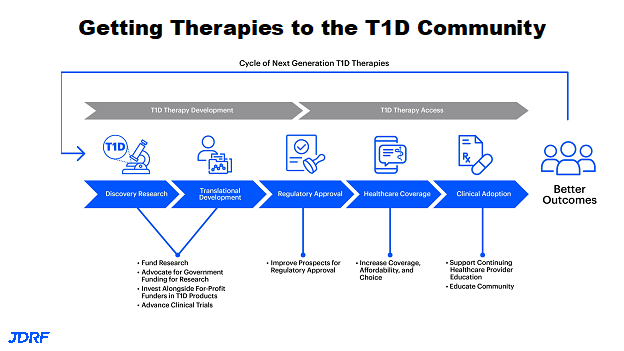
This article will cover the top advances from July 1, 2021 to the present, as presented by Sanjoy Dutta, Breakthrough T1D Chief Scientific Officer May 2022. This research talk was presented on May 11th for Breakthrough T1D research information volunteers. Each area of research will be covered with basics, and then one or two advances for this year in that area. Breakthrough T1D continues to strengthen all of the scientific work in T1D (Type 1 Diabetes) across the pipeline. Breakthrough T1D has been part of taking scientific ideas all the way to having people with diabetes do even better, including those that are at risk of getting T1D. Breakthrough T1D not only has strength within research they fund, they also have strength in being able to leverage expertise outside Breakthrough T1D to assist in the research pipeline moving smoothly. This process is a cyclic process, meaning as we learn how people with diabetes are doing with these new therapies we can also improve on the concept and bring ideas into the discovery research so we can continue to develop advanced products. Examples of this would be having developed better closed loop systems. There will be advances in the near future also.
There are many areas where Breakthrough T1D has had RFAs (Request for Applications). These areas include better screening tools, immunotherapy approaches on how to preserve the cells once they are implanted for graft preservation, improving glucose monitoring, and adding keto monitoring functionality. There are also RFAs in psychosocial and mental health areas, such as with eating disorders. To avoid complications in the field of research we are adding new RFAs for cardiovascular disease and kidney disease. The Breakthrough T1D research portfolio supports all stages of T1D, and is also able to specifically support young investigators interested in making T1D their field of research. Breakthrough T1D not only has innovative grants presented at postdoctoral events, but also provides career development awards.
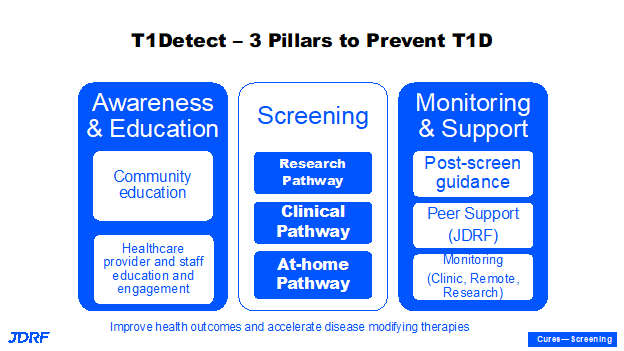
The first project area to be covered is screening. It is important to screen for risk of T1D. There needs to be more education, monitoring, and support provided for screening. The T1Detect screening program puts together educational awareness for the need for screening, for the act of screening the results of autoantibody tests, and it also provides a support system. The last point is important in terms of tests that provide results and additional glucose control support monitoring related to screening, as well as the psychosocial support system. This program has been supported by multiple sponsors and more of these are to come. This has helped to expand the program in a very short time. There are three main pillars in T1Detect which is a general population screening currently available in the United States. The first is the T1Detect system itself. Second is that screening is part of the research pathway to preventing and curing T1D, such as through TrialNet and other programs nationally and globally. We can have both TrialNet and T1Detect as ways to detect autoantibodies. We need to adopt all possible measures so we can identify these individuals. T1Detect is important, as most who are diagnosed with T1D do not know of any family connection.
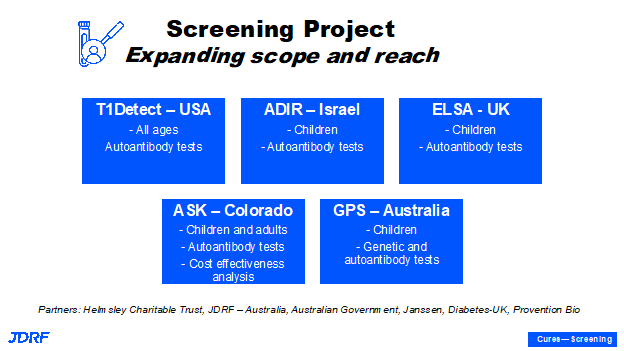
TrialNet is an important study of those who do have a family connection to T1D. The third pillar is that we have to have awareness and education around what T1D is. This provides knowledge about the disease, and can help provide a softer landing when individuals are diagnosed with the disease, along with education to the general population and healthcare providers. Once someone is given a diagnosis of autoantibody positivity, there is support that can be provided. This tool can help in monitoring progression and with encouragement and willingness to participate in clinical trials. We can also accelerate the development of disease modifying therapies by screening. T1Detect provides a general population screening of all ages and all socioeconomic strata. There are also programs Breakthrough T1D supports in the state of Colorado as well as in other countries like Israel, the United Kingdom, and Australia. Breakthrough T1D also plans to expand further screening approaches. It is important to have a strong screening program in the US and globally. One main reason to screen for T1D is to be able to avoid diabetic ketoacidosis (DKA), which can be acute and cause hospitalization and there are long term benefits if individuals can avoid going into DKA when diagnosed with T1D.
The goal of disease modifying therapies is to develop therapies that can change the course of a disease. In autoimmune T1D, the immune system is inflamed and starts destroying the only cells that make insulin, which are the beta cells. The cells progressively get stressed. There is a lot of debate as to which happens first: is it the immune system that has gone awry and is destroying the beta cells, or is it that the beta cells are unhealthy and progressively dying which alerts and triggers the immune system? Regardless, when you have unhealthy beta cells they are progressively dying, and the question is: how can we convert them into healthy beta cells? This will need to be addressed by either rebalancing the immune system, or preserving and regenerating the last beta cells in the pancreas. This can be applied to every stage of the disease. Breakthrough T1D is primarily focused on the at-risk population and those recently diagnosed with T1D. Breakthrough T1D does this by supporting clinical trials for new therapies.
INNODIA is another successful public-private partnership that involves working together to reach the goal of curing T1D. This includes 5-7 pharmaceutical companies and biotech companies and about 20+ research institutions. Breakthrough T1D and Helmsley Charitable Trust are putting their resources and brainpower together to create this consortium that can screen individuals at risk, identify the newly diagnosed people, and have interventional clinical trials. There are about four or five clinical trials right now that are testing individual therapies, such as the blood pressure lowering medication Verapamil or a transplant medication that has been used for about 60 years called ATG (anti-thymocyte globulin), as well as many others. Breakthrough T1D is beginning to embark on combination therapies to test drugs that have complementary mechanisms. These can turn down the immune system, but also promote beta cell survival. Breakthrough T1D will continue to support initiatives such as INNODIA and many others. Clinical Trials are happening today because of the way Breakthrough T1D funded research. Getting to this point involved significant resources, significant time, lots of patience, and learning a lot along the way. That is what culminated in the clinical trials. Breakthrough T1D has a good pipeline of therapies and research that is being funded and in preclinical development. If you go back and look at the pipeline, these therapies are turning down or turning off the autoimmune system here and rebalancing it, therefore promoting survival of beta cells, regrouping beta cells, or transforming other types of cells into beta cells that can produce insulin. These new therapies are in different stages of preclinical testing. We need all types of therapies since T1D is a very heterogenous disease, meaning there are several ways that the disease may have occurred. The disease is also not the same for everyone.
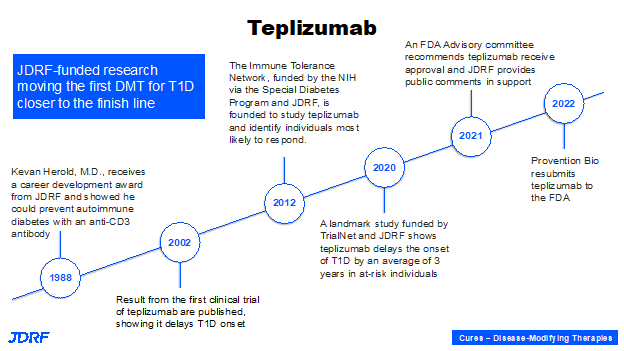
Another example of beta cell therapies within disease modifying therapies to either slow down, halt, or delay the progression of T1D is a collaboration with Dr. Maike Sander, University of California San Diego and Ionis Pharmaceuticals, which is not a traditional T1D company. She has developed an agent that can make beta cells proliferate, but we need to deliver it safely with no unwanted effects. Ionis Pharmaceutical has a unique technology that can help direct a therapeutic, sometimes called a payload, into a tissue of interest. In the case of T1D, the target is the beta cells. This collaboration is being currently tested in mouse models. With success in mouse models, the next step will be human clinical trials. This is an example of preclinical research that is very important today, so it can go into clinical testing in human clinical trials. A good example of the trajectory of preclinical research to therapy is the drug teplizumab, which is shown to prevent stage 3 clinical diabetes for three years. Breakthrough T1D started with a small trainee award to Professor Kevin Harold, when he was at University of California San Francisco in the lab of Jeffery Bluestone. From the beginning concept, to understanding a disease pathogenesis, to developing a therapy and making sure it is effective (meaning efficacious and safe) is a long process which takes many years. The final step needs a pharmaceutical that can take it on and bring it to the stage for FDA review and hopefully a favorable decision.
JDRF’s fingerprint, support, and advocacy is here throughout the process, whether by directly funding it, or by advocating for Special Diabetes Program (SDP) resources through the Immune Tolerance Network and TrialNet, to fund these studies. There is also the T1D Fund investing in Provention Bio that has teplizumab currently under review at the FDA. There is a lot of promise there and it will be the first disease modifying therapy. We know how to develop a therapy that can change the course of a disease; the process is not going to take another 30 years to develop another disease modifying therapy. It will become more of a seamless process for other therapies that are going to follow suit. It is important to remember that years and decades of research are important for us to cross that threshold and then success will follow.
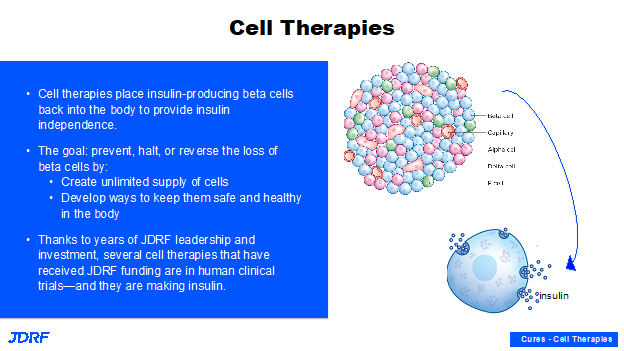
We have seen a lot in the news on cell replacement therapies and how you can make these beta cells outside the body and put them back in the body. The cells being transplanted also have other cell types that contribute other critical functions in the pancreas. The goal here in the development pathway is to create these beta cells outside of the body from stem cell populations, and then to demonstrate that they are faithfully producing insulin, that they are reproducible, and that they can be scaled up in large quantities.
During twenty years of research starting with Professor Doug Melton’s lab with Breakthrough T1D leadership, the safe and ethical use of stem cells is bearing fruit today. We have multiple sources of stem cell-derived beta cells, and they are currently in clinical testing. Breakthrough T1D is now working to protect these beta cells so they can last a long time and do their job of providing insulin independence and good glucose control in people with diabetes. Breakthrough T1D has multiple teams, the research team, regulatory colleagues in DC all working very hard to not only identify ways of protecting these beta cells, but also to see how this can go through a regulatory pathway and move forward in clinical testing and ultimately receive approval.
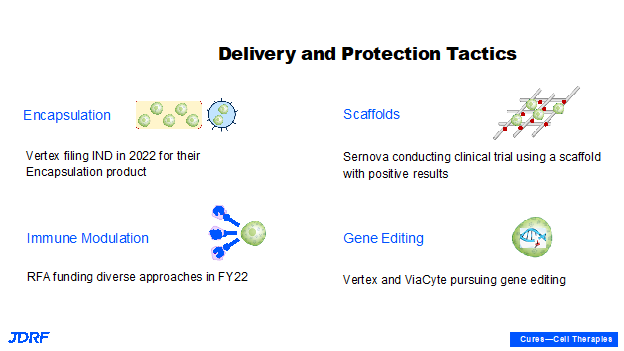
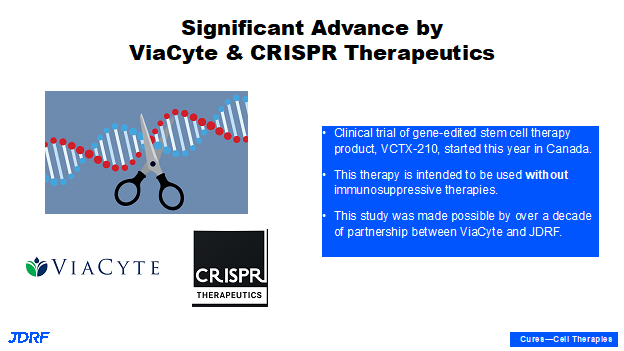
This is a technical slide showing different ways of protecting the beta cells. Breakthrough T1D is currently supporting all of these approaches. Sometimes one approach can be combined with another. For example, gene editing technology could be combined with local immune-modulation. Another example is that in this particular fiscal year with encapsulation, Vertex announced that they plan to file a new drug application to test their encapsulated stem cell-derived product in humans by the end of 2022. This is coming up very soon and with scaffolds we have multiple ongoing clinical trials with Sernova. There are also requests for applications for finding different ways of providing local immunosuppression. Currently with cadaveric allo-transplantation, there is entire body immunosuppression to allow the graph to survive. There is also work being done to make better beta cells, such as cells that do not trigger the immune system and that do not subsequently require immune suppression. Theoretically, an individual would not require suppression when using encapsulation and scaffolds, as these cells will be able to escape the internal immune system. We are aspiring in FY22 with these new and exciting technologies. Both Vertex and Viacyte announced their gene editing technologies moving forward. Viacyte has even started testing in a clinic in Canada. The first patient has been dosed with the gene edited stem cell product and if successful, this will not require immunosuppression. We are currently awaiting these results.
This slide shows the collaboration with CRISPR that is providing the gene-editing technology. This CRISPR technology won the Nobel Prize for Medicine several years ago. This is a technology that a lot of other disease areas are now tapping into, especially for diseases that have end organ failure. The hope is that this technology can help regenerate organs that can be transplanted without the need for immunosuppression, as is usually required for any transplantation.
Breakthrough T1D is also funding several Breakthrough T1D Centers of Excellence that are working on research and product development in this area. These are located in New England, Northern California, Michigan, and Canada at the University of British Columbia. They are all addressing different components of cell replacement therapy, whether when modifying immune cells so that they can be supportive of beta cells when transplanted, or when trying to provide a healthy metabolic environment for the survival of these grafted beta cells (such as is being done at University of Michigan); as well as most recently at the University of British Columbia where stem cell products are being manufactured, producing larger quantities and enhancing immune regulation. There will be a collaborative approach with what we learn from all of the different centers in regard to this research.
This slide gives a birds-eye view of what we are doing with the company Vertex today. In the early 2000s, Breakthrough T1D gave a grant to Professor Doug Melton to create the first insulin-producing cells from stem cells. He had multiple years meeting key milestones and being able to make these cells, and enabling them to be produced in larger quantities. Dr. Melton formed a small biotech company called SEMMA to be able to start on the path of becoming a product. The T1D Fund invested in this company with Vertex then acquiring SEMMA. This product also has the fast-track designation from the FDA, recognizing the unmet clinical need in this field. Vertex has released data over the last six or seven months and the most recent data is that the clinical trial has shown efficacy and safety with the first individual. This individual is fully insulin-independent with a half dose of these cells. There has been data released with a press release noting the second and third patients are doing well, showing efficacy and safety. This trial is happening in Canada. It is currently on hold in the US.
In the area of Breakthrough T1D’s improving lives portfolio, there has been a lot of movement as Breakthrough T1D supports the development of devices, drugs, behavioral interventions for better glucose control, better metabolic control, prevention of progression of complications, and a combination of all of the above to help with the burden of diabetes. Breakthrough T1D has also been active in advocating for lower insulin pricing in the United States. Breakthrough T1D, along with about 13 other like-minded partners, has the philanthropic goal of making sure that this life saving medication is available and accessible to everyone, including and especially underinsured and uninsured people.
We are partnering with Civica, a not-for-profit company, that will be working on three most commonly prescribed insulins in the United States. This project will develop biosimilars for glargine (Lantus), lispro (Humalog), and aspart (Novolog). When they are available anyone will be able to purchase insulin at no more than $30/vial and $55.00/box of five pens. While Civica is developing these biosimilars, Breakthrough T1D continues to be very effective at advocating for Congress to address the cost of insulin so it is affordable for all. Breakthrough T1D advocacy in Washington, DC, along with many thousands of Breakthrough T1D volunteer advocates, are constantly advocating for continued research funding while we fund research that can make these insulins that are used by millions of people around the world, and needed by 1.6 billion Americans in the United States.
Breakthrough T1D continues to make progress in the area of artificial pancreas systems. Recently, The Omnipod 5 system became available on the market. This is a really a big moment for pump users who do not like having tubing on their pump. This is currently the only tubeless artificial pancreas in a closed loop system. This was released with amazing data in both adults and children and was approved right away at the same time as it was for adults. These are the advances we are seeing today because of the generous Breakthrough T1D leadership over the last 15 years creating and supporting research along with creating regulatory pathways. The first basic research for an AP system was funded by Breakthrough T1D along with many other leaders in the field. The main algorithms were developed within the Breakthrough T1D artificial pancreas consortium. There are more therapies and improvements for AP systems coming out in FY22. There is a continual process of checking in with those with T1D, seeing how they are doing, and then going back to further innovate and discover, which leads to enhanced products.
There is now a seven-day infusion set from Medtronic. The Tandem pumps remote blousing is amazing. It works for both Android and iOS users. The implantable sensor from Senseonics called Eversense in now approved. There are many people that like this particular sensor and it has amazing accuracy. We have now gotten use to these advances, which is great. Breakthrough T1D is still working to make them a fully closed loop system. Work still needs to be done in making devices even smaller and more user friendly, particularly for those who are independent in our population.
Breakthrough T1D continues to work on the mental health burden of living with T1D. There is significant investment being done now in psychosocial and behavioral medicine. Our colleagues have put together several funding opportunities. Breakthrough T1D has been working on diabetes distress in young adults, this year with emphasis on the spectrum of eating disorders. Whether it is subclinical or a preclinical eating disorder where an individual does not have one yet, but with an intervention that can stop the progression to a clinical eating disorder. Work is being done with several clinical eating disorders in those with T1D and are ongoing in the psychosocial portfolio. It is helpful that these therapies can be delivered fairly easily through telemedicine. There are also options for in-person visits. These telemedicine visits continue to work during times of pandemic while people are remote. Breakthrough T1D insists that these clinical trials ensure recruitment from the underserved population. The investigators are encouraged and required to enroll a certain percentage of the population that are underserved or underinsured, and those in the lower socioeconomic strata.
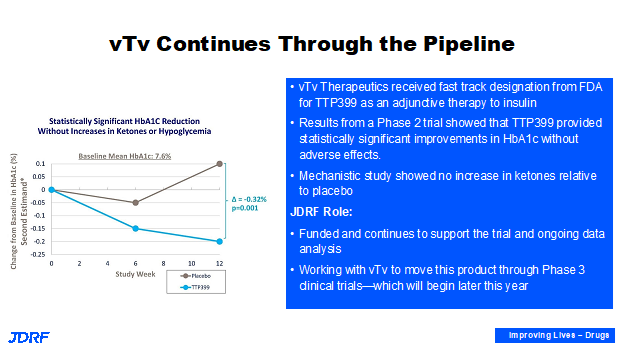
vTv Therapeutics has an oral drug that affects the glucose that is in the blood after a meal injection. This works in the liver. Breakthrough T1D has partnered with vTv Therapeutics. It has been demonstrated in a phase two study that it lowers A1c as well as provides other glucose benefits. Recently, Breakthrough T1D also did a mechanistic small study to demonstrate that it has these benefits without increasing hyperglycemia or ketosis. Breakthrough T1D will continue to work with vTv both with research and regulatory leadership in Washington, DC to advise them on the design of a phase three trial for T1D. Support for these companies is needed so therapies are realized by individuals with T1D.
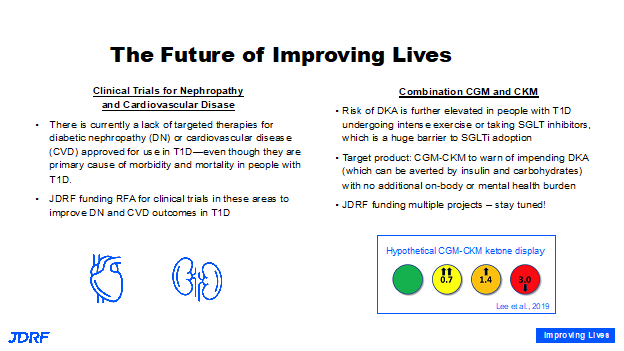
The good news is that the level of complications for someone with T1D has gone down, but it has not gone away. For many, good glucose control remains out of reach. Unfortunately, people with T1D are still vulnerable to having kidney function loss, vision loss, and heart attacks. It remains to be the highest cause of mortality in people with T1D. Breakthrough T1D is making a very big push here. For many current clinical trials for heart and kidney disease, T1D is often an exclusion criterion. This means there needs to funding for clinical trials for those with T1D to develop new therapies addressing heart and kidney disease in this population. There is no therapy that is specific for kidney or heart disease approved in T1D, but there are therapies that are approved for type two diabetes, and for people without diabetes but who have kidney disease. We have to make these trials happen to bridge the gap. One of the therapies that shows tremendous efficacy is SGLT inhibitors. This has had limited use in T1D. There has been a great deal presented on the possible benefits of SGLT inhibitors for those with T1D. Researchers are working on added risks with these drugs concerning DKA. Additional work has been launched for the development and support of continuous ketone monitors that work in combination with continuous glucose monitors that will help. People can reap the benefits of the SGLT inhibitors for glucose control, body weight loss, cardiac protection, and renal protection. In general, it can benefit many with T1D.
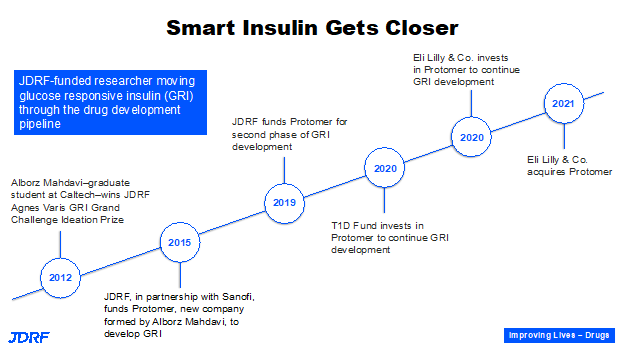
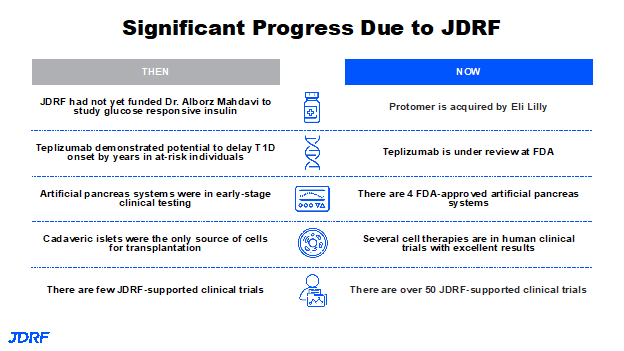
In 2012, Dr. Alborz Mahdavi, Caltech, won a Junior Grand Champions prize for his first idea of developing a glucose responsive insulin, or a smart insulin. He has progressively received support from Breakthrough T1D and other partners, such as Sanofi Pharmaceuticals; eventually the T1D Fund invested in this. This idea became Protomer Technologies, which was acquired by Eli Lilly. This puts it into the hands of a company that has 100 years of experience with insulin. Glucose responsive insulin developed by Protomer has ongoing clinical trials that we have seen in the news and will actually help us realize the dream of a smart insulin in the not-too-distant future. Breakthrough T1D is helping the multiple players that are coming to the table to get to a therapy that can go to the FDA for approval. This is the last step before a therapy can be available to patients.
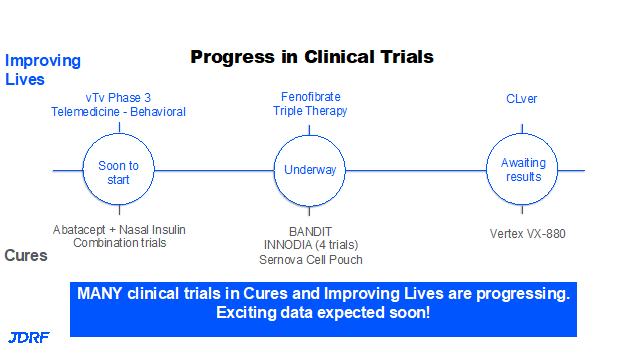
In the not-so-distant past, there were not as many clinical trials for therapies for T1D. Today we have over 150 clinical trials that are actively recruiting just in the US for T1D. Over 50 plus trials are being supported directly by Breakthrough T1D, some in partnership with other funders and sometimes entirely by Breakthrough T1D. Breakthrough T1D’s team of volunteers is helping to educate and share information about how to participate in clinical trials, the importance of clinical trials, and the fact that these clinical trials are safe. Often people do better with their glucose control during a clinical trial, even in the placebo group, because of the medical attention they receive and because of the regimen of the study. This is the time to learn more about drug trials and ask questions and engage our community in participating in clinical trials across all three stages of T1D. We can have more therapies very soon if we have participation in clinical trials. Here are more examples of the progress that is being made because of clinical trials:
We are waiting on the results of the CLVer study, which is fully enrolled. It is a combination of using a hybrid closed loop system in kids recently diagnosed with T1D and the blood pressure drug Verapamil. This study is looking at protecting the beta cell from the stress environment during the prolonged honeymoon period. The results of the study are expected very soon. Another clinical trial using fenofibrate is also underway. These trials are a combination addressing glucose and metabolic control along with functional therapy. There are multiple trials that both Breakthrough T1D and colleagues, such as INNODIA in Europe are supporting. There is a trial using a JAK inhibitor in Australia that will be of interest. In cell therapy, there are about four or five clinical trials going on with new therapies or novice cell pouches. Breakthrough T1D is also supporting trials that are about to go to print on the prevention of T1D using a combination of an immunotherapy, along with nasally delivered insulin. Then there is the trial with Vertex VX-880. The trial is currently on hold with the FDA and when that hold is removed the trial can be completed. The other great tool that was developed several years ago is the Breakthrough T1D Connection Tool. This involved the work of staff and volunteers working hand in hand with IT members. It is very easy to use this matchmaking tool to see if you are eligible for a clinical trial; it will show your choices within a given radius that you select. It is quick and easy. Test it out and consider participating in a clinical trial. It will take a very long time to get new therapies and cures without participation in clinical trials. Breakthrough T1D will continue to keep our community informed by keeping everyone abreast of breaking news through our insider blogs, chapter, and national newsletters or social media.
In summary, there was no funding in the area of glucose responsive insulin 10 years ago and now we have one or more clinical trials ongoing. We have large pharmaceutical companies interested in our efforts that have added funding. What would be the first disease modifying therapy to be approved, teplizumab, is under review at the FDA. It is anticipated that the decision will come sometime this summer. With AP systems today, we are moving from cadaveric islet cell transplantation to now having four or five trials, and testing stem cell-derived beta cells in the clinic. Breakthrough T1D was funding about 10 clinical trials 12 or 13 years ago; Breakthrough T1D is now funding over 50 in their portfolio and around 150 active trials across the country. It is great to look into all of the clinical trials that are available.
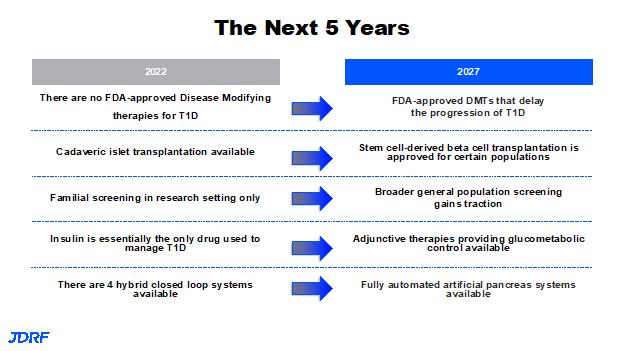
There are many hopes for the rest of 2022 and the next five-year timeframe. One is to have the therapy teplizumab approved. We are looking forward to seeing other therapies coming out. There should be advancements with development of stem cell-derived insulin, stem cell-derived beta cell products that are in advanced clinical development, and potential approval for the beta cell transplantation such as the one Vertex is doing currently. In the screening program, we should have more robust information so that broader general population screening gains traction. Work will be done within the goal of a five year timeframe. Breakthrough T1D hopes to get screening programs covered and are working on getting a plan in place. This starts with improving education, and monitoring awareness to go along with realizing our goal of population-based screening adjunctive therapies. In the United States the only adjunctive therapy that is approved is Pramlintide. There needs to be more so more people can see better glucose control with metabolic benefits, long term kidney protection, and heart protection. Breakthrough T1D knows we will get there and they will not be complacent. There is still work to be done such as making devices easier to use without causing scar tissue. Work in the future will continue to reduce the size of these devices, especially the pump, along with being able to use sensors longer. These areas will also continue to be a focus.
This is a recap of the research talk done by Dr. Sanjoy Dutta, May 2022. If you have questions about clinical trials please contact me, Debbie Evans. I am the Clinical Trials Education Volunteer (CTEV) for the Minnesota and Dakotas Chapter. I can be reached at debbieaevans1@gmail.com.
Here is the link to the current clinical trials in our area.