
The short version
Breakthrough T1D’s newest publication outlines what the future of beta cell replacement therapies looks like—and how we can make these therapies a reality for everyone with type 1 diabetes (T1D) who wants them through innovative clinical trial design and expanding the pool of eligible trial participants.
Breakthrough T1D, in collaboration with other leading experts in the field, recently published an article titled “Future Directions and Clinical Trial Considerations for Novel Islet β-Cell Replacement Therapies for Type 1 Diabetes” in the journal Diabetes. Breakthrough T1D Research and Advocacy staff who contributed to the publication include Sanjoy Dutta, Ph.D., Chief Scientific Officer, Esther Latres, Ph.D., Vice President of Research, and Marjana Marinac, Pharm.D., Associate Vice President of Regulatory Affairs.
Beta cell replacement therapies have the potential to cure type 1 diabetes (T1D) by removing the need for external insulin. While donor islet transplantation can result in insulin independence and results from early trials with manufactured islets are encouraging, people with T1D need better tools and therapies. This publication serves as a roadmap for the entire field—including researchers, product developers, industry, regulators, clinicians, and people with T1D—to address these needs and ensure that beta cell replacement therapies can get to people with T1D as quickly and safely as possible.
Read on to learn more about what the future of beta cell replacement therapies looks like.
Key Takeaways
- Currently available beta cell replacement therapies are limited to people with T1D with unstable blood sugar management, typically measured by HbA1c levels, in addition to dangerous lows (hypoglycemic events) that require immediate assistance. These therapies also require lifelong immunosuppression.
- New and improved beta cell replacement therapies are on the way, and we need to make sure that they are available for people with T1D beyond those with unstable blood sugar management and severe hypoglycemic events.
- Clinical trials for these therapies must be strategically designed to include more people with T1D given the potential for insulin independence.
- Shared decision-making between people with T1D and their care team will be critical for evaluating the risks versus benefits of a beta cell replacement therapy, especially as more options become available.
Where we are now
People living with T1D depend on external insulin to manage their condition throughout their lives. Despite advancements in diabetes technology, such as continuous glucose monitors (CGMs) and automated insulin delivery (AID) systems, most people with T1D are unable to achieve blood sugar targets, rendering them at a higher risk for complications, reduced quality of life, and lower life expectancy.
It’s simple: we need to do more for the T1D community.
One promising avenue to meet these needs is through cell replacement therapy. Lantidra®, the first FDA-approved donor-derived islet replacement therapy for T1D, has proven to be safe and effective in eliminating severe hypoglycemia, providing insulin independence, and improving quality of life. However, this therapy is limited to people with severe hypoglycemia and requires immunosuppression to prevent rejection of the transplanted cells, which can have side effects. Even more, these donor-derived islets are in limited supply.
Alternative beta cell replacement therapies are emerging—and we can no longer limit their development to people experiencing severe hypoglycemia.
As stated in the publication:
Given the proven benefits of islet transplantation extending far beyond the amelioration of severe hypoglycemia that has been documented and the understanding of the risk profile gained over the past 20 years, consideration must be given to broadening the application for islet beta cell replacement.
Developing cell therapy strategies to meet unmet needs of the T1D population
Breakthrough T1D’s vision for the T1D community includes beta cell replacement therapies with no immunosuppression that are available to everyone who wants them. We are committed to making this a reality through our Project ACT (Accelerate Cell Therapies) initiative, which will accelerate the development of these therapies to achieve our vision as quickly as possible.
Project ACT
Scientific progress takes time, resources, collaborations, and effort. To accelerate islet replacement therapies faster than ever, Breakthrough T1D launched Project ACT (Accelerate Cell Therapies) to simultaneously advance research, development, regulatory policies, access, and adoption of manufactured islet therapies that do not require broad immunosuppression.
We are entering an exciting era of beta cell replacement. Emerging therapies are addressing challenges such as cell source and scalability, resulting in the development of islets derived from sources other than donors, including manufactured islets and porcine islets. Up-and-coming therapies are also testing different transplantation sites, methods of delivery, and cell protection strategies to prevent immune rejection with the fewest side effects possible (and ideally no immunosuppression). Learn more about what scientists are doing to optimize beta cell replacement therapies.
Breakthrough T1D’s continuous support of many of these therapies, such as Vertex’s manufactured islet therapy zimislecel, has been critical to accelerating them through the clinical pipeline. Explore emerging beta cell replacement therapies in clinical trials now—and see how Breakthrough T1D’s commitment to these therapies helped make this possible.
When successful, the advent of new, safe, scalable, and effective beta cell replacement therapies will provide the T1D community with options. As these therapies are moving their way through the pipeline, we need to ensure they are being studied in a broader T1D population who stand to benefit.
So, how do we make this happen?
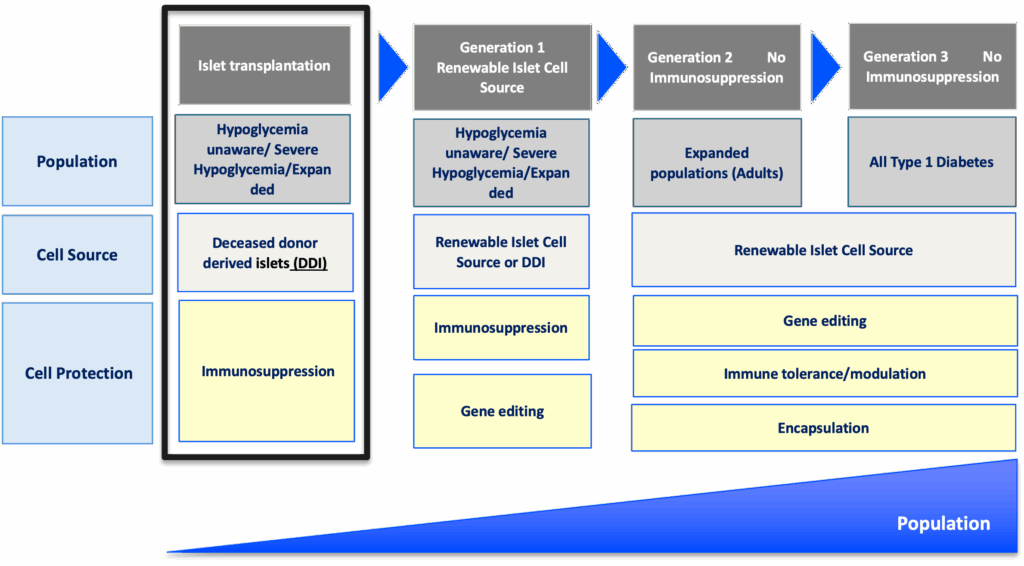
The roadmap for emerging beta cell replacement therapies
The goal
Accelerate availability of emerging next-generation beta cell replacement therapies for every person with T1D who wants them by designing clinical trials that speed their development, regulatory approval, access, and adoption.
Expanding the T1D population eligible for beta cell transplantation
Current trials testing beta cell therapies necessitating immunosuppression require participants to have elevated HbA1c levels and recurrent severe hypoglycemic events. This limits the pool of participants to people who meet the requirements and are most likely to benefit, given the side effects associated with chronic, broad immunosuppressants.
Clinical trials for emerging beta cell replacement therapies should broaden eligibility criteria so more people with T1D can participate—and experience the potential benefits. When designing new clinical trials, sponsors and regulators should consider including a broader range of HbA1c levels, clinically important or serious hypoglycemic events, and other complications.
Studies have found that the T1D community is generally open to beta cell replacement therapies as a potential solution to T1D, and people are willing to accept the associated risk versus benefit considerations for the possibility of becoming insulin independent. A Breakthrough T1D assessment also found that physicians are interested in recommending beta cell replacement therapies to people with T1D—especially if they don’t require immunosuppression.
“Clinical trials to support the development of islet cell replacement therapies need to evolve to include a broader representation of people living with T1D who could benefit from these novel therapies. This includes expanding the outcomes used to assess the benefits of cell replacement that reflect how people with T1D feel and function.”
Marjana Marinac, Pharm.D., Associate Vice President of Regulatory Affairs

Placing people with T1D at the center of clinical trial design
The outcomes used to assess the effectiveness of cell therapies currently in clinical trials, including those involving deceased donor islets, are acceptable for emerging beta cell replacement therapies. These include on-target HbA1c levels, absence of severe hypoglycemia, significant reduction or elimination of external insulin, and restoration of the body’s insulin production as measured by C-peptide.
Other endpoints that should be considered include CGM metric targets like time-in-range in addition to person-reported outcomes. Understanding how a beta cell therapy may affect a person’s health-related quality of life—such as diabetes distress, fear of hypoglycemia, or social and family dynamics—will be critically important for calculating the risk to benefit ratio of these therapies.
Read more about why person-reported outcomes are important for cell therapies.

“There are still significant unmet needs in the T1D community. Breakthrough T1D’s roadmap is supported by the assessment of clinically meaningful outcomes and driving research toward solutions that address key factors such as cell sources and protections strategies that will broaden the people with T1D who could benefit from emerging cell replacement therapies.”
Esther Latres, Ph.D., Vice President of Research
Innovative trial designs to accelerate development of cell therapies
Clinical trials for beta cell replacement therapies are generally based on a single-arm, open-label design—meaning there is no placebo group and both participants and researchers know which therapy is being administered. While this design can work for emerging beta cell therapies, single trials with multiple arms testing alternative transplant sites, immune protection strategies, or other methods have the potential to speed up the pace of development.
Similarly, adaptive trial designs use mid-trial interim analyses of study data to inform the remainder of the trial. This helps researchers learn what’s working (or not working) and adjust the design accordingly, with guidance from regulatory agencies, so the rest of the trial is a focused and efficient use of time and resources. Potential interim changes to trial design include reducing the number of participants required, eliminating doses, recruiting people who are most likely to benefit, or stopping the trial outright due to clear success or failure.
By applying guidance in therapeutic development and innovative trial designs to emerging beta cell replacement therapies, we can move early-stage trials along faster, thereby allowing regulators to make decisions sooner. To support quicker trials and reduce the possibility for delays, researchers, developers, and regulators around the world need to work together to achieve convergence on trial populations, endpoints, and innovative designs that will meet regional requirements.
Learning from past successes
People with T1D continue to live with unmet needs with still significant risk for long-term complications, and they need more therapeutic options. Right now, most clinical trials for beta cell replacement therapies requiring immunosuppression are limited to a small portion of the T1D population. This needs to change—especially given the potential for insulin independence. The T1D community must be put first when making decisions about beta cell replacement therapies, and Breakthrough T1D is making sure that this happens.
Adjusting how we approach clinical trials for emerging beta cell replacement therapies will be critical for ensuring we accelerate the research, development, regulatory approval, access and adoption of these novel therapies. Breakthrough T1D successfully accomplished this for AID systems—and we are confident that following a similar roadmap for cell therapies will get us to the finish line, faster.
The journey of AID systems
Learn more about the critical role of Breakthrough T1D in driving AID systems forward, recounted by Breakthrough T1D volunteer Doug Lowenstein.
Curative therapies for T1D are in reach. This roadmap, in conjunction with our Project ACT initiative, is key to bringing beta cell replacement therapies to every person with T1D who wants them.
To make this a reality, everyone needs to work together. As stated in the publication, “This requires a comprehensive strategy and a coordinated collaboration across stakeholders in every field relevant to islet cell replacement.” This roadmap is a guide for moving toward our common goal of a cure for T1D as soon as possible.
Breakthrough T1D will continue to lead the way until the T1D community can choose the beta cell replacement therapy that works best for them, regardless of blood glucose management or hypoglycemia status. Everyone deserves the chance to benefit.