
Breakthrough T1D and ISPAD Announce 2022 Drash Fellows
Breakthrough T1D and the International Society for Pediatric and Adolescent Diabetes (ISPAD) are thrilled to announce the 2022 recipients of the Allan Drash Clinical Fellowships—and they’re an incredible bunch. They are: Muzna Arif, Pakistan Moomin Hussain Bhat, India Emine Ayça Cimbek, Turkey Hiba Elshafie, Sudan Elif Eviz, Turkey Berna Eroğlu Filibeli, Turkey Sara Gafar, Sudan […]

The Innovative Insulin Your Kids Can’t Use…Yet
What if it was safe and effective to take a rapid-acting inhaled insulin in children/teens as they start eating? The INHALE-1 clinical trial is finding out.

Top Scientists Unite at the EASD Annual Meeting
Leading researchers gathered for the annual meeting of the EASD, at which more than 30 studies were presented by Breakthrough T1D researchers.

EASD Meeting Brings Together World-Renowned Minds in Diabetes Research
Leading researchers from around the world will gather for the annual meeting of the European Association for the Study of Diabetes (EASD).

Type 1 Diabetes Champion Susan Solomon Passes Away
Breakthrough T1D mourns the passing of Susan Solomon

Glucagon: Your Friend in Low Places
Glucagon. It’s a word familiar to people with T1D, yet one most hope to avoid discussing. But what is glucagon and what is its role in the management of T1D?
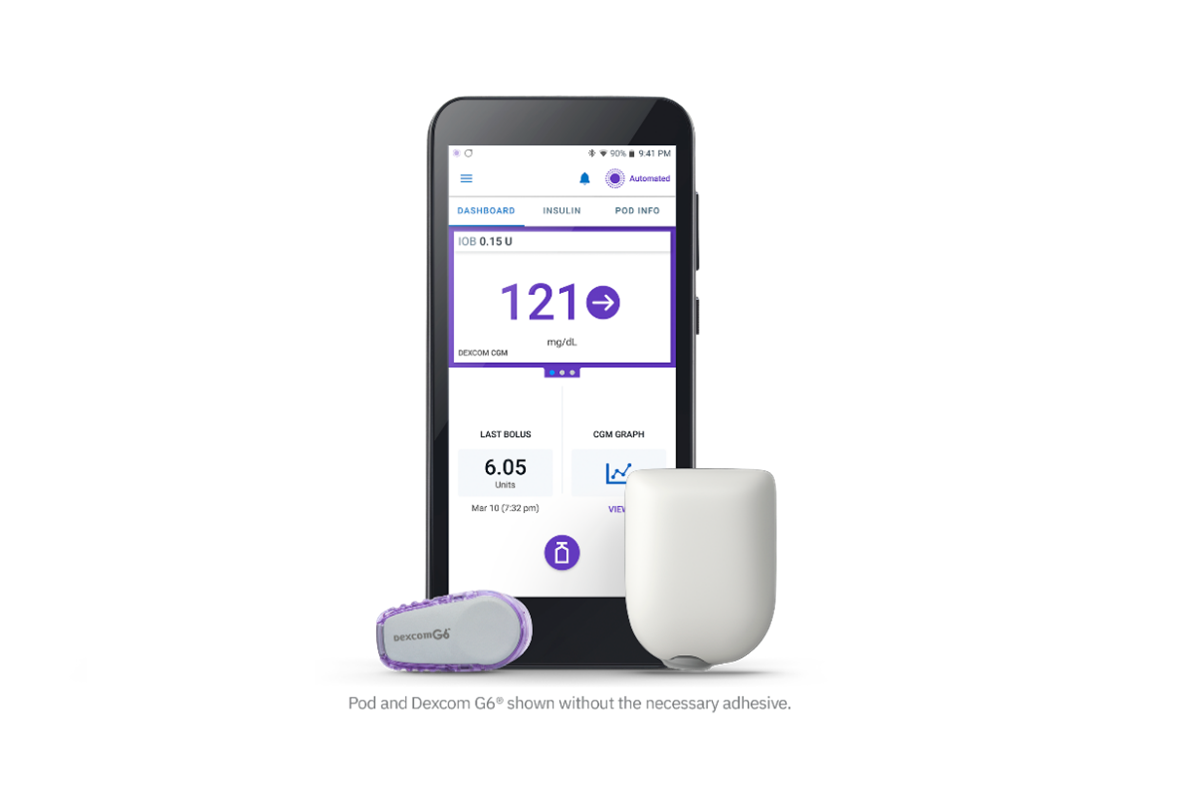
FDA Authorizes Omnipod 5 for Ages 2+ in Children with Type 1 Diabetes
The FDA authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system, for children 2+, using an algorithm that began by Breakthrough T1D funding.

Study Shows No Link Between T1D Diagnoses and COVID-19
Breakthrough T1D-funded researchers in Colorado and Germany found no link between COVID-19 and type 1 diabetes in children and adolescents ages 1-18.

A New Tool to Accurately Diagnose Type 1 in Adults
Misdiagnosing adults with type 1 diabetes (T1D) is an all-too-common problem that can have tragic consequences. Breakthrough T1D and IQVIA partnered up to tackle it—and the results were just published.

Diabetes Research in Pediatrics? The International Society Has Your Back
Learn more about the recipients of the International Society for Pediatrics and Adolescent Diabetes (ISPAD)-Breakthrough T1D Fellowships here!