
Want to Stave Off Type 1 Diabetes? Beta Cell Proliferation May Be the Key
In Nature Metabolism, Breakthrough T1D-funded researchers—including the lead author, Ercument Dirice, Ph.D., and senior author, Rohit Kulkarni, M.D., Ph.D.—found an unidentified piece of information: By enhancing beta cell mass secondary to a robust beta cell proliferation early in life, they could protect mice from developing type 1 diabetes (T1D). I’ll say that again: By enhancing […]
A DIY approach to artificial pancreas technology
Discover how the DIY approach to artificial pancreas technology is empowering people with type 1 diabetes.

A Win for the European T1D Community: Zynquista (sotagliflozin) Approved for Type 1 Diabetes
In a second win for the type 1 diabetes (T1D) community, the European Commission has approved Zynquista™ (sotagliflozin) for use in adults with T1D with a body mass index (BMI) above or equal to 27 kg/m2, in combination with insulin to improve blood-sugar control. It and Forxiga® (dapagliflozin and marketed in the United States as […]

End-stage kidney disease: A possible biomarker of 10-year risk—and therapeutic target—for people with T1D
In the United States, diabetic kidney disease, or DKD, is responsible for more than half of all new cases of end-stage renal (kidney) disease. What’s more, over the last few decades we saw improvements in all major diabetic complications, but end-stage kidney disease rates declined the least, and it is still a cause of premature […]

What is nPOD?
The human pancreas is very difficult to study, as Breakthrough T1D-funded scientists well know. It cannot be imaged or safely biopsied from a living person. As a result, much of the research on how Type 1 Diabetes (T1D) occurs was previously conducted in animal models. While these studies taught us a great deal, they did […]


European Commission Has Approved Forxiga for Adults with Type 1 Diabetes
It’s official: The European Commission has approved Forxiga® (dapagliflozin and marketed in the United States as Farxiga®) for adults with type 1 diabetes (T1D), when used in conjunction with insulin to improve glycemic control. This is the first oral medicine for T1D in Europe, and blocks SGLT, which is responsible for glucose absorption in the […]

Mini, AP Patch Given FDA Nod for Fast-Track Review
A closed-loop patch insulin pump/CGM system has been granted FDA Breakthrough Device Designation by the FDA. The designation is designed to provide a faster track to FDA approval for devices that are considered to provide novel treatment options. The device was created by EOFlow, a company based in South Korea, and the technology was funded […]

The 20th Anniversary of the Edmonton Protocol
This day, 20 years ago, a person with type 1 diabetes (T1D) was cured. Well, pseudo-cured. On March 11, 1999, the Edmonton Protocol had its first participant. This ground-breaking clinical trial was testing a new type of islet transplantation, without corticosteroid immunosuppressive drugs (which is necessary for the prevention of graft rejection). The seven people […]
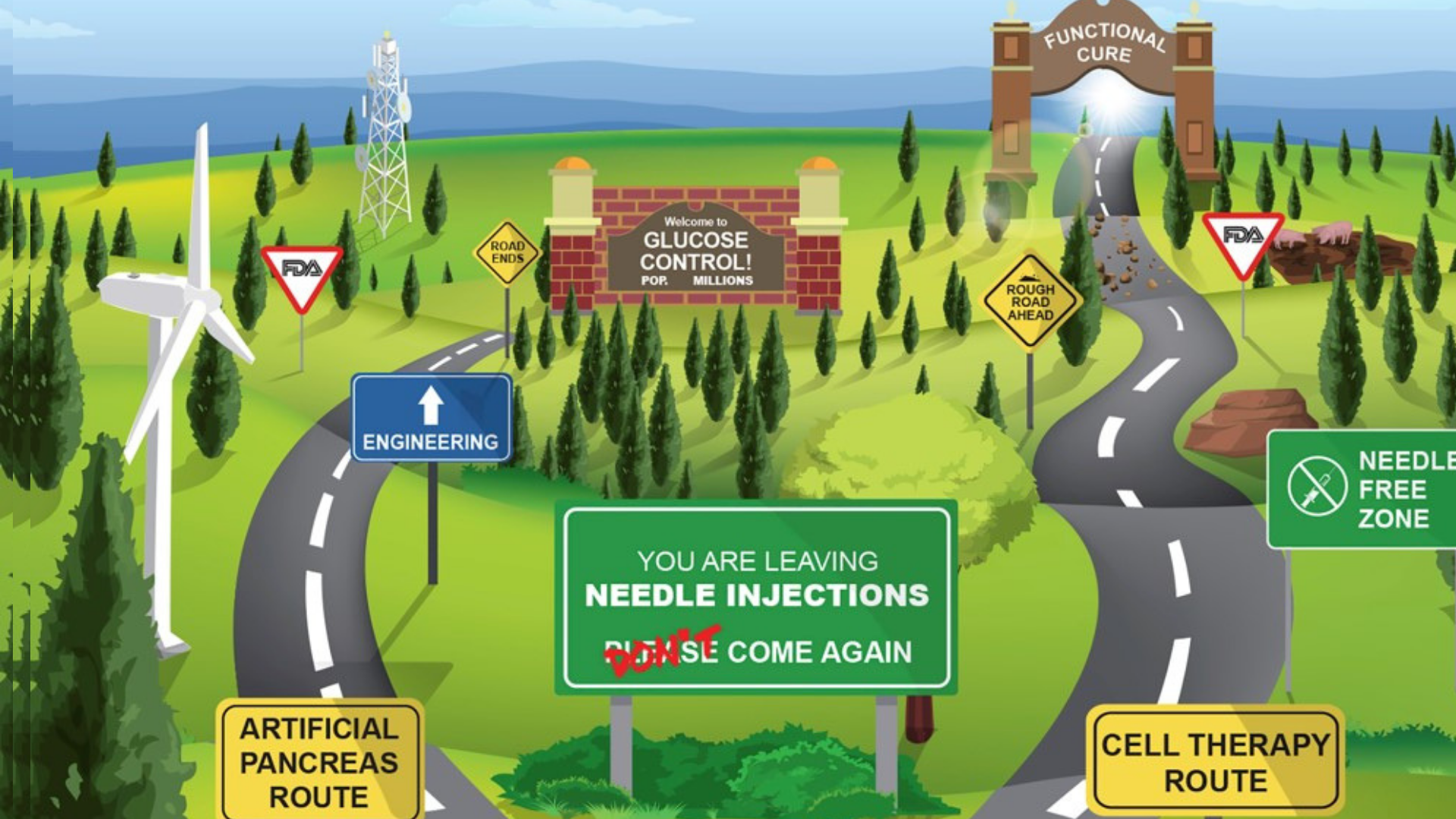
Insulin Discovered Nearly 100 Years Ago: How Far We’ve Come and the Road Ahead
Incredible strides have been made since the discovery of insulin almost 100 years ago. Insulin formulations have improved dramatically, blood-sugar levels can be measured continuously and first-generation artificial pancreas systems have reached the market. Yet only a small percentage of people with type 1 diabetes (T1D) achieve their blood sugar goals. In a review published […]