
Cell therapy moves forward across the pipeline
Beta cell replacement therapies aim to overcome the obstacles to realize their potential, but several approaches are making them a thing of the past.
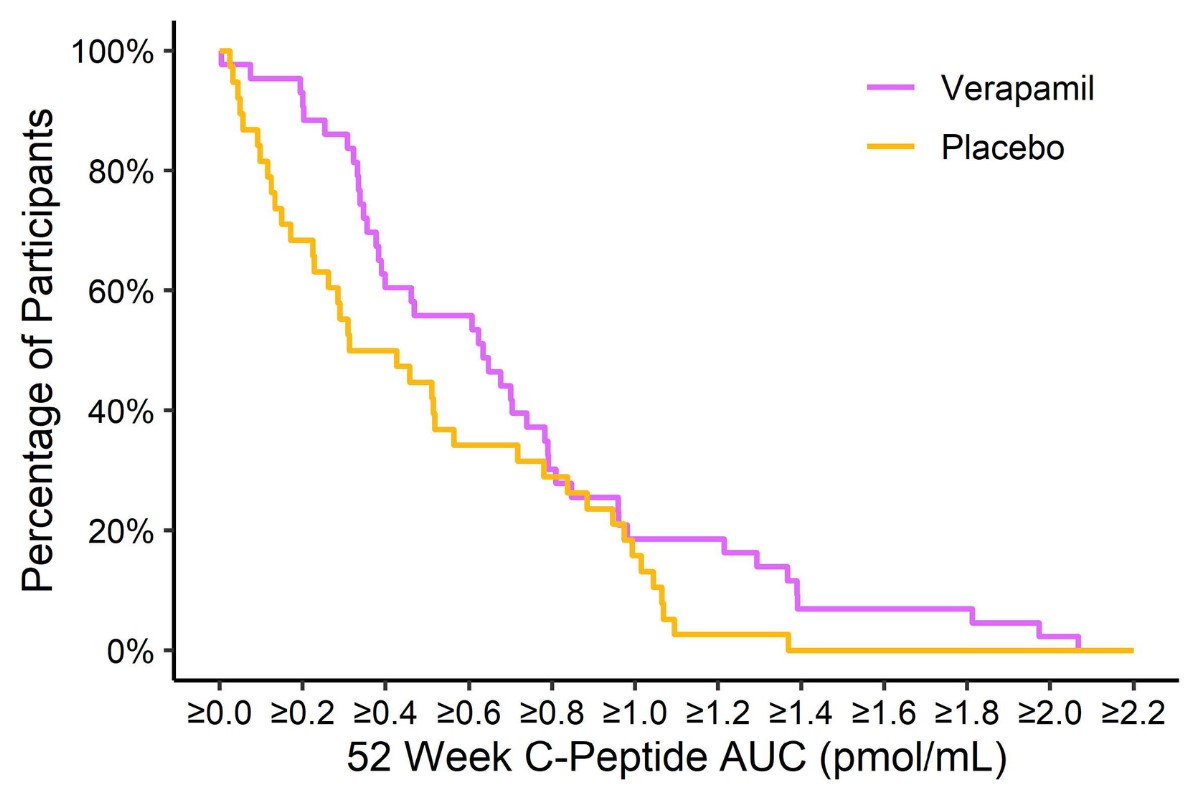
Verapamil Slows Type 1 Diabetes Progression in Newly Diagnosed Children and Teens
The CLVer study found that newly diagnosed individuals on verapamil were making more insulin one year after diagnosis than those on placebo.

ATTD Conference Brings World-Renowned Minds to Berlin, Germany
This year’s Advanced Technologies & Treatments for Diabetes (ATTD) meeting, held February 22-25, will have 45 presenters who are or were Breakthrough T1D-funded.

The Innovative Insulin Your Kids Can’t Use…Yet
What if it was safe and effective to take a rapid-acting inhaled insulin in children/teens as they start eating? The INHALE-1 clinical trial is finding out.

Diabetes Research in Pediatrics? The International Society Has Your Back
Learn more about the recipients of the International Society for Pediatrics and Adolescent Diabetes (ISPAD)-Breakthrough T1D Fellowships here!

Breakthrough T1D Timeline: Stem Cell Therapies
Stem cells have the potential to lead to treatments for type 1 diabetes, but it has taken Breakthrough T1D more than 20 years to get to this point.

At ADA, Breakthrough T1D-Funded Research Takes Center Stage (with a “Happy Hour” by Dr. Aaron Kowalski)
At the ADA’s Scientific Sessions, Breakthrough T1D scientists presented on some of the most important topics, all with the same goal: Ending type 1 diabetes.

Breakthrough T1D Heads—In Person!—to ADA’s 82nd Scientific Sessions
From June 3-7, diabetes researchers around the world will gather—in person!—at the American Diabetes Association’s 82nd Scientific Sessions. #JDRFxADA

100, 100: Ending Diabetic Eye Disease, Forever
Breakthrough T1D has supported eye disease research since its beginning, and has driven discoveries that have reduced the risk of blindness by 95%.

Top Researchers Gather for the 15th ATTD Conference
Leading researchers gathered, in person, for the annual meeting of the ATTD Conference (April 27-30), featuring 30+ studies presented by Breakthrough T1D scientists.