
ATTD Conference Brings Together Top Minds in Diabetes Research
Diabetes researchers from all over the world gather for the ATTD Conference, which will take place in-person and online from April 27-30 in Barcelona.

100, 100: Breakthrough T1D Research Leads to a New Class of Glucose-Lowering Agents
You’ve seen the ads: Victoza®. Ozempic®. Trulicity®. But did you know that these drugs came about, in part, because of type 1 diabetes research?
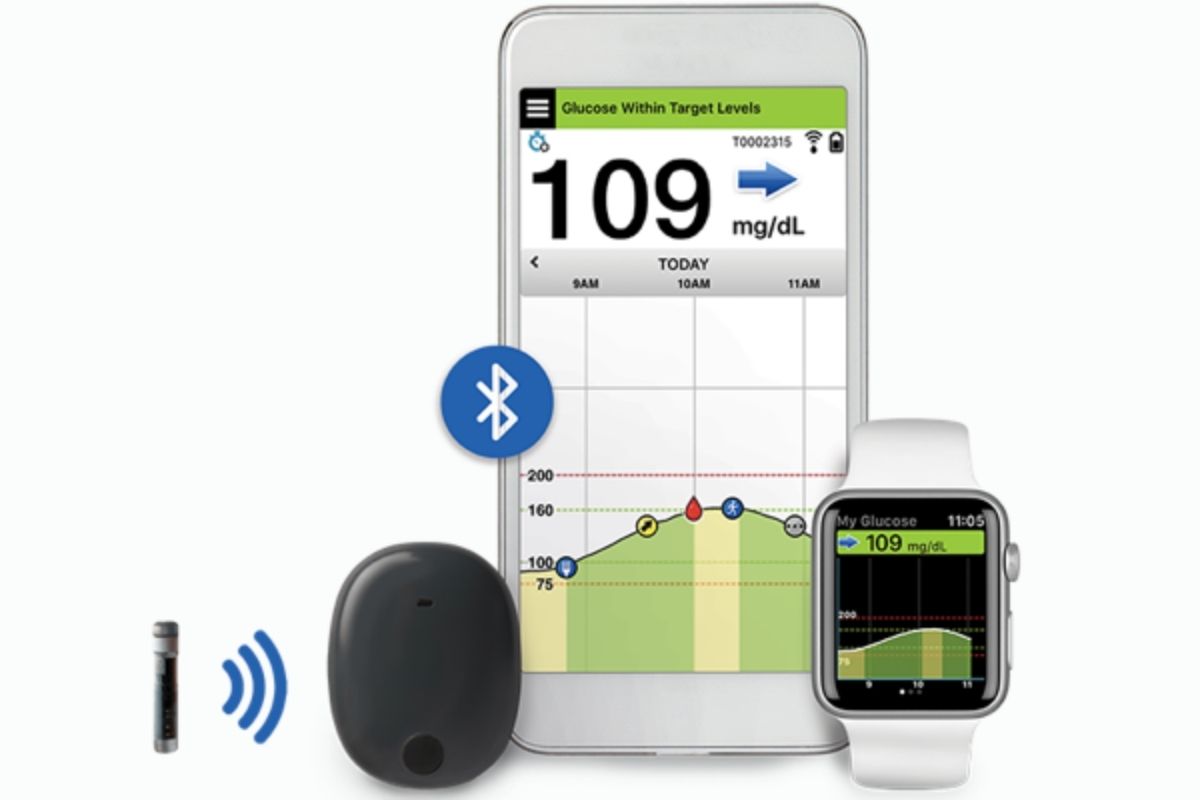
FDA Approval of Senseonics Eversense® CGM for Use for Up to 6 Months
FDA approves Senseonics Eversense® E3, the first long-term implantable CGM system, and the E3 includes technology that extends the use for up to 6 months.

100 Years, 100 Breakthrough T1D Scientists: The BB Rat—An Animal Model of Type 1 Diabetes
In 1974, a colony of non-diabetic BB rats develops diabetes. Examination of their pancreases revealed that they had no beta cells.

Interview with our New Community Screening and Clinical Trial Education Director
Breakthrough T1D has a new community screening and clinical trials education director. She's been a volunteer and now staff since her daughter was diagnosed in 2002.

Insulin: A Story of Innovation
On January 11, 1922—100 years ago—14-year-old Leonard Thompson, who was diagnosed with diabetes 3 years earlier, became the first person to receive insulin.
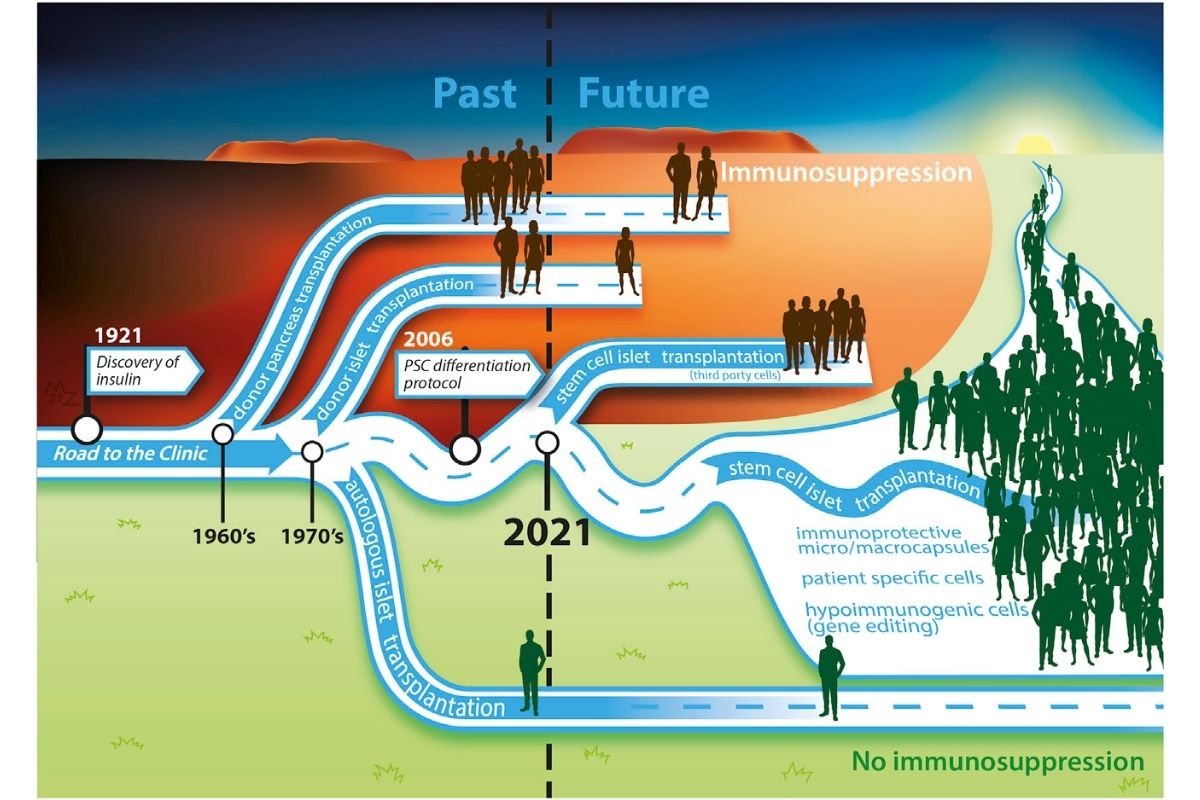
Stem Cell Replacement Therapy: Its Time has Begun
ViaCyte has results on its second stem cell-based technology, providing evidence of stem cells secreting insulin in response to meal consumption.

Progress Toward Cures: Thoughts at Year’s End from Breakthrough T1D’s CEO
Breakthrough T1D CEO Aaron Kowalski, Ph.D., weighs in on the exciting news surrounding stem cell-derived beta cell replacement therapies, now in human clinical trials.
First-in-Human Gene-Edited Stem Cell Trial? Check.
ViaCyte, a beta cell replacement company long supported by Breakthrough T1D, and CRISPR Therapeutics have a new first: Gene-editing for type 1 diabetes.
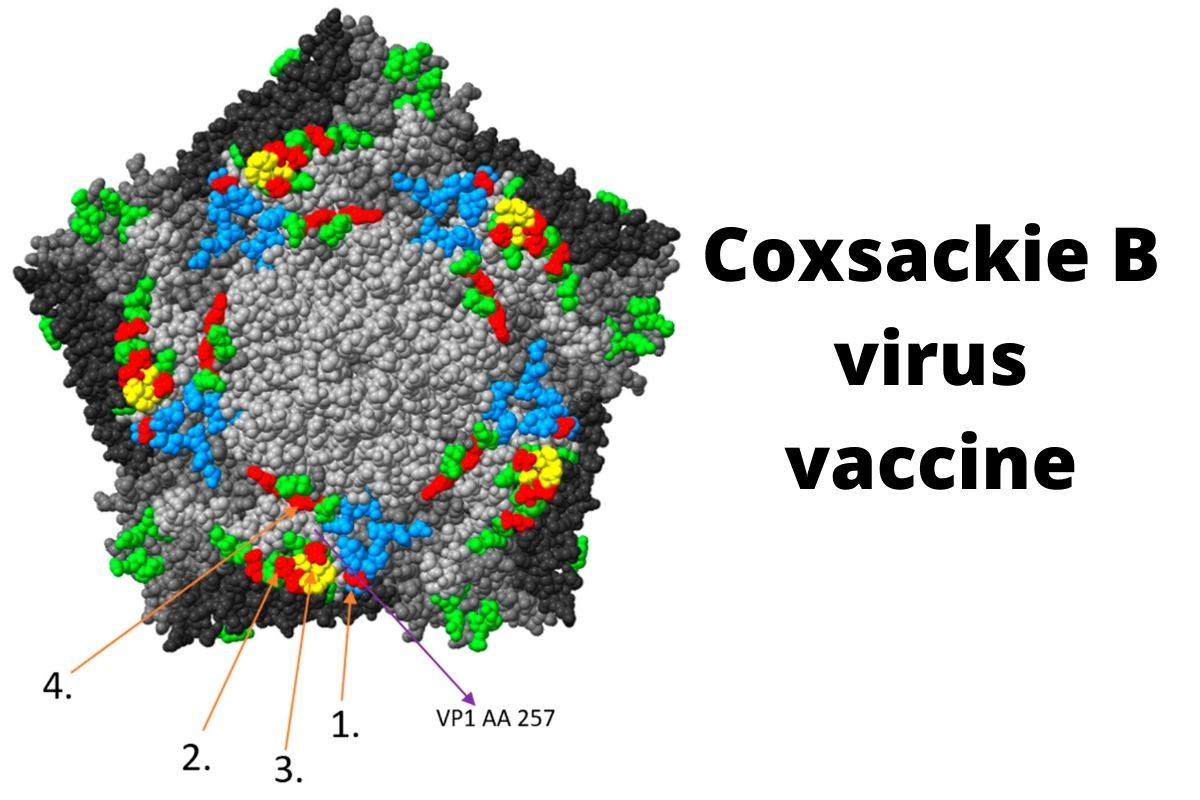
First-in-Human T1D Vaccine Trial Reports Positive Results
A first-in-human study of the coxsackie B vaccine—made by Breakthrough T1D-funded researchers Drs. Hyoty and Knip—reports positive results.