Grant Beard, Executive Operating Partner, LP, and Co-investor at Blue Point Capital Partners, will serve as Breakthrough T1D International Board of Directors (IBOD) Chair, succeeding current Chair Joseph (Joe) P. Lacher. Michelle Griffin, a management consultant with more than 25 years of experience, will serve as Breakthrough T1D IBOD Vice Chair, succeeding current Vice Chair Lisa Fishbone Wallack.
Grant Beard: Supporting Breakthrough T1D’s Research Mission
 Grant and his wife Susan have been volunteer leaders since their daughter Emily’s T1D diagnosis in 2006 at age 14. They were recognized for their support of T1D research and research related education with the 2016 Jane Jospey Cobb Promise Award.
Grant and his wife Susan have been volunteer leaders since their daughter Emily’s T1D diagnosis in 2006 at age 14. They were recognized for their support of T1D research and research related education with the 2016 Jane Jospey Cobb Promise Award.
Grant has served eight years on the IBOD and sits on the Breakthrough T1D T1D Fund Board of Directors. As part of the IBOD, he chairs the Audit & Risk Committee and is a member of the Funding Committee. Grant previously served as the President of the Board of Directors for Breakthrough T1D’s Metro Detroit/Southeast Michigan Chapter (now the Michigan and Northern Ohio Chapter).
“Working with my colleagues on Breakthrough T1D’s International Board of Directors to drive mission impact has been a deep honor for me,” Grant said. “Michelle and I, in partnership with our fellow Breakthrough T1D leaders, will continue to move our mission forward. Today, given where the science is, we have an unprecedented opportunity to make a difference in the lives of those living with T1D–here and around the world.”
Michelle Griffin: Supporting T1D Families
 Michelle’s son Cameron was diagnosed with T1D in 2007 at age nine and she and her husband, Tom Parker, have been actively involved with Breakthrough T1D since.
Michelle’s son Cameron was diagnosed with T1D in 2007 at age nine and she and her husband, Tom Parker, have been actively involved with Breakthrough T1D since.
Michelle served on the IBOD for six years and was Chair of the Development and Audit Committees. She is currently a member of Breakthrough T1D’s Global Mission Board and Audit Committee and is a Special Advisor to the Funding Committee. She has worked in a variety of roles for Breakthrough T1D’s Northern California Chapter, presently as Co-Chair of Leadership Giving. In 2017, Michelle received the Jim Tyree Chairman’s Choice Award for her noteworthy volunteer contributions to Breakthrough T1D.
“I have been a Breakthrough T1D true believer ever since our son Cameron participated in a Breakthrough T1D funded clinical trial two months after diagnosis in 2007,” Michelle said. “It is an incredible honor to be asked to serve as Vice Chair, especially at this time when we are seeing the fruits of the Breakthrough T1D community’s longtime support of our mission to accelerate life-changing breakthroughs. I am very excited to partner with Grant, our talented staff, and passionate volunteers to keep the momentum going as we get ever closer to making cures a reality.”
This new leadership is a positive evolution for Breakthrough T1D, which has recently seen a number of exciting research breakthroughs.
“In the past few months alone, we have seen incredible progress in cures research—most notably in cell therapies,” said Aaron J. Kowalski, Ph.D., Breakthrough T1D Chief Executive Officer. “Having Grant and Michelle step into these leadership roles ensures we will continue to drive our mission forward globally through scientific advancements and advocacy.”
“We thank Joe and Lisa, who began their leadership in the early months of the pandemic, which upended our fundraising model. Under their direction, Breakthrough T1D devised strategic priorities to advance more therapies, raise more funds, and activate more volunteers,” Kowalski added. “We welcome Grant and Michelle, and look forward to partnering with them to amplify our impact as we improve lives around the world and move closer to curing T1D. It is truly an exciting time to be at Breakthrough T1D.”
About the Breakthrough T1D International Board of Directors (IBOD)
IBOD is the governing body for Breakthrough T1D and is tasked with accelerating the organization’s mission progress toward life-changing breakthroughs to cure, prevent, and treat type 1 diabetes and its complications. Learn more about Breakthrough T1D’s volunteer and staff leadership.
During our 50+ years of advancing diabetes research, Breakthrough T1D has funded the best and the brightest investigators. But which of them have contributed the most to what we know about type 1 diabetes (T1D) today? In honor of the 100th anniversary of the first administration of insulin, Breakthrough T1D is launching “100 Years, 100 Breakthrough T1D Scientists,” the story of the scientists who contributed to discoveries that played a part in the vast knowledge that we have about diabetes today.
The first up: Gian Franco Bottazzo, M.D. Born in Venice, he obtained his M.D. degree in 1971 at the University of Padua. Shortly after, he began a research fellowship with immunologist Deborah Doniach, M.D., at Middlesex Hospital, and, in 1998, he returned to Rome. He died in 2017.
Aha! An Autoimmune Disease!
When Breakthrough T1D was founded, there were no types of diabetes. There was no type 1, no type 2. It was all diabetes.
But, early on in Bottazzo’s fellowship, in 1974, he discovered—for the first time—that diabetes is associated with the development of islet cell antibodies (ICA), leading to a landmark publication in the field.1 This was instrumental in establishing type 1 diabetes (T1D) as an autoimmune disease.
The discovery of ICA introduced a new era of diabetes research and understanding:
1. A chronic disease: Together with Andrew Cudworth, M.B., and funded with a Breakthrough T1D grant, Dr. Bottazzo measured ICA in samples from relatives of individuals with T1D. In 1981, they reported that ICA was detectable years before the clinical onset of disease,2 a finding that eventually led to the notion that T1D was a chronic disease.
2. Before clinical symptoms: Moreover, ICA were shown to have value in identifying individuals who would later develop T1D, providing new staging for presymptomatic T1D. It also enabled the discovery of more antibodies that were associated with T1D. There are now five antibodies—ICA, GAD65, IAA, IA-2, and ZnT8—connected with the disease. The presence of two or more means that your lifetime risk of getting T1D is nearly 100 percent. Dr. Bottazzo’s research contributed to a number of studies about the genetic risk of developing T1D.3,4
3. Refine the tests for T1D-related antibodies: These were quantified by Breakthrough T1D units,”5,6 a measure of the number of T1D-specific antibodies that were detected in a person. This led to the formation of a number of clinical studies to determine the risk of T1D—DAISY, ASK, and TrialNet in the United States, BABYDIAB in Germany, DIPP in Finland, and DiPiS in Sweden.
More recently, Breakthrough T1D initiated T1Detect, an education and awareness program to expand screening to the general population. The program’s aim is to make people understand what type 1 is, how screening is advantageous to the public, how they can be involved, and what to do if you are positive for T1D-specific antibodies.
4. Conduct clinical trials: It also enabled clinical trials aimed at delaying the clinical onset of diabetes, before symptoms appear. This includes teplizumab—a drug that blocks CD3, a blood marker that helps activate immune cells. It was the first study to significantly delay the onset of T1D, for nearly 3 years, in individuals at-risk of developing the disease.
I hope you enjoyed reading about a T1D champion. Follow up each week to find out who we selected and their major discoveries, in honor of the 100th anniversary of the first administration of insulin.
Research is how insulin was discovered as a therapy for diabetes, and Breakthrough T1D research is advancing us toward cures and the next generation of life-changing breakthroughs for T1D.
- Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30; 2 (7892): 1279-83. PMID: 4139522.
- Gorsuch AN, Spencer KM, Lister J, McNally JM, Dean BM, Bottazzo GF, Cudworth AG. Evidence for a long prediabetic period in type I (insulin-dependent) diabetes mellitus. Lancet. 1981 Dec 19-26; 2: 1363-1365. doi: 10.1016/S0140-6736(81)92795-1. PMID: 6118756.
- Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12; 2 (8359): 1115-1119. doi: 10.1016/S0140-6736(83)90629-3. PMID: 6138647.
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8; 313 (6): 353-60. doi: 10.1056/NEJM198508083130604. PMID: 3159965.
- Bonifacio E, Bingley PJ, Shattock M, Bean BM, Dunger D, Gale EA, Bottazzo GF. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990 Jan 20; 335: 147-149. doi: 10.1016/0140-6736(90)90013-U. PMID: 1967440.
- Gleichmann H, Bottazzo GF. Progress toward standardization of cytoplasmic islet cell-antibody assay. Diabetes. 1987 May; 36 (5): 578-584. doi: 10.2337/diab.36.5.578. PMID: 2436962.
We have many reasons to celebrate in the type 1 diabetes (T1D) community. First and foremost, we celebrate YOU. Your support of our efforts is inseparable from the tremendous progress we’ve seen in accelerating cures, improving lives, and advocating for people with T1D and their loved ones.
We celebrate the impact and influence that you have made in research and advocacy to make a difference for members of the T1D community every single day. Here are some examples of how far we’ve come toward creating a world without type 1 diabetes.
1. Accelerating Cures with Stem Cell-Based Therapies
Ninety-one percent less insulin, at half the target dose? Check.
Clinical trial for a gene-edited stem cell replacement therapy? Check.
First reported evidence of implanted stem cells secreting insulin in response to meal consumption? Check.
We’ve got a lot to be thankful for in stem cell therapies.
This year, The New York Times and Good Morning America highlighted the story of Brian Shelton, the first person to receive Vertex’s stem cell-derived therapy, VX-880, showing he needs 91 percent less insulin than before the transplantation. While these are early results from just one person, and this therapy is only being tested in people with severe hypoglycemia, breakthroughs like this bring us a step closer to finding cures for T1D.
Starting in 2000, Breakthrough T1D played a key role in the development of VX-880 by awarding Douglas Melton, Ph.D., a grant to make insulin-producing beta cells from stem cells, which his team did in 2014. Melton founded Semma Therapeutics in 2015, and the Breakthrough T1D T1D Fund made a catalytic investment in 2017. Vertex acquired Semma for almost $1 billion in 2019, and, in March 2021, the FDA fast-tracked its trial of VX-880—producing these exciting results just six months later.
Another big player in the stem cell-derived beta cell space is ViaCyte, who is a longstanding Breakthrough T1D partner and had two announcements. One is for VCTX210, which combines CRISPR Therapeutics’ gene editing technology with ViaCyte’s proprietary stem cell therapy, which will launch a clinical trial in Canada—the first for a gene-edited cell replacement therapy in T1D. The second is preliminary results from its second technology, VC-02™, which yielded the first reported evidence of implanted stem cells secreting insulin in response to meal consumption.
2. Coxsackie Vaccine Has Positive Results
Breakthrough T1D has been funding research into the role viruses play in the development of T1D since the late 1970s and, in the 1990s, we funded a postdoctoral fellowship for Heikki Hyöty, M.D., Ph.D., who demonstrated that enteroviruses—specifically coxsackie B—are the main culprit for T1D. Coxsackie B is a common pathogen where, in most circumstances, the infection is asymptomatic or results in mild symptoms. (Coxsackie B, however, in rare cases, may also lead to viral meningitis, heart or brain infection, or hand, foot, and mouth disease.)
Hyöty co-founded Vactech in 2001, who developed PRV-01, a vaccine targeting coxsackie B, and licensed it to Provention Bio*, which got an investment from the Breakthrough T1D T1D Fund for a first-in-human clinical trial. The results: Not only was it well tolerated, but it induced high concentrations of anti-coxsackie B antibodies.
This was only the interim results; the final results will be revealed this year. But, if they are the same as the interim results, continued development of the vaccine and its use to reduce the burden of T1D will be under way.
*Provention also has teplizumab, the first drug to delay the onset of T1D for nearly three years, under review by the FDA. Breakthrough T1D had a hand in the development of teplizumab from almost the beginning. We gave a Career Development Award to Kevan Herold, M.D., who had just started his faculty-level career at The University of Chicago, in 1988-1990. He showed, in an early animal study, that he could prevent autoimmune diabetes with an anti-CD3 antibody (which, later, became a humanized version, teplizumab). He has gone on to receive more than 15 grants from Breakthrough T1D, and was the lead on the clinical trial that demonstrated that it could delay the onset of T1D for nearly three years.
3. COVID-19 Update
Many of you joined our efforts to raise awareness of the impact that COVID-19 has on the T1D community. This work resulted in the U.S. Centers for Disease Control and Prevention (CDC) updating its guidelines to include T1D on the list of medical conditions associated with higher risk of becoming severely ill from COVID-19. It also helped us secure for members of the T1D community prioritized access to the COVID-19 vaccines and boosters when they first became available.
4. On Medicare? Accessing CGMs Just Got Easier
A regulation by the Centers for Medicare and Medicaid Services (CMS) puts in place expanded access to continuous glucose monitors (CGMs) for people on Medicare. The final rule provides coverage for all FDA authorized CGMs. Previously, CMS only covered therapeutic CGMs, which are those devices approved by the FDA to make insulin dosing decisions (e.g., without use of fingersticks in addition to CGM). This expansion means that people on Medicare with diabetes will now have access to a broader choice of CGMs, similar to what is available to those with commercial insurance.
The positive impact of CGMs on the health and well-being of those with T1D is clear and Breakthrough T1D has long advocated for broader coverage and choice of CGMs, resulting in private plan coverage starting over a decade ago, and Medicare coverage on a partial basis in 2017 and expansion last year. The CMS decision marks an important milestone for Medicare’s coverage of therapies that will improve the lives of those with T1D.
5. Regulatory Approval of Several T1D Therapies and Technologies
We fund research to facilitate the development of new therapies and technologies to make day-to-day life with T1D easier, safer, and healthier, until we can find cures or prevent this disease. In 2021, we got a lot to be thankful for.
Apps
- The FreeStyle® Libre 2 iOS app received approval from the FDA for adults and children ages 4 and up with diabetes. This app is used with the Abbott FreeStyle® Libre 2 CGM system, which was approved on June 15, 2020. Prior to this approval, FreeStyle® Libre 2 users had to wave a reader over the glucose sensor. Now, they can use their iPhone instead.
- The Bigfoot Unity™—a Bluetooth-enabled insulin pen cap—received FDA clearance for individuals 12 and older. For the first time, people who use multiple daily injections (MDI) to manage their diabetes will be able to integrate with a continuous glucose monitor and get personalized insulin dosing recommendations, lessening the burden of T1D.
Insulin
- The FDA approved Semglee® (insulin glargine-yfgn), a long-acting insulin, as the first ever interchangeable biosimilar insulin product, for adults, children, and teens with T1D (and adults with type 2 diabetes). It is biosimilar and interchangeable to Lantus®, a long-acting insulin, and may now be substituted for the prescribed reference product (Lantus®) at the pharmacy counter.
- Walmart will sell the ReliOn™ label insulin, NovoLog® (insulin aspart), a rapid-acting insulin, and NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart), a rapid-acting insulin plus intermediate-acting insulin that works up to 24 hours, for use in adults and children with diabetes. The cost is $72.88 for 10mL insulin vials or $85.88 for a package of five FlexPens®.
- The FDA approved an expanded label for the rapid-acting insulin, Lyumjev® (insulin lispro-aabc injection) 100 units/mL, for use in insulin pumps for people with diabetes. (It was first approved by the FDA in June 2020.) As a rapid-acting mealtime insulin, Lyumjev helps control blood-sugar levels after meals in adults with diabetes.
Infusion Set
- The FDA has approved Medtronic’s Extended Wear Infusion Set (EWIS), which is the first infusion set approved for seven days. No other infusion set is currently approved for more than three days.
Hypoglycemia
- The FDA approved Zegalogue® (dasiglucagon) injection, for the treatment of severe hypoglycemia in children and adults with diabetes aged 6 and above. Its approval was based on results from three clinical trials, showing an average time to blood sugar recovery of 10 minutes following injection. This is the fourth FDA approval of a glucagon treatment for severe hypoglycemia, providing people with choice for which Breakthrough T1D strongly advocates.
6. Artificial Pancreas Systems: Three at the FDA
With your support, we have ushered in therapy innovations that have made managing T1D easier and have led to improved health outcomes.
The most noticeable impact is in the influx of diabetes devices that have made T1D easier to control and have freed people from the constant worry about their disease. A significant milestone—or three to be exact—is that three artificial pancreas systems—Tidepool Loop, Omnipod 5, and Medtronic MiniMed™ 780G—have been submitted for FDA review.
The Tidepool Loop is an iPhone app that brings together an insulin pump, CGM, and automated insulin dosing algorithm that adjusts to the user’s basal rates as often as every five minutes and allows the wearer to bolus from the phone. The Omnipod® 5 System is the first and only tubeless artificial pancreas system, which pairs its disposable insulin pump (which you can change every 3 days) with a Dexcom G6 CGM. And the Medtronic MiniMed™ 780G is an update on the 670G, which was approved in 2016, and has a time-in-range of around 75 percent.
7. A Potential First-in-Class Adjunct Therapy
The FDA granted Breakthrough Therapy designation for vTv Therapeutics’ TTP399 as an adjunct therapy to insulin for T1D. Breakthrough Therapy designation is intended to expedite the development and review of drugs for serious and life-threatening conditions.
In a Breakthrough T1D-funded phase II clinical trial called Simplici-T1, TTP399 significantly improved HbA1c in people with T1D. Additionally, trial participants who received TTP399 showed a reduction in insulin dose, reduced hypoglycemia, and no increase in diabetic ketoacidosis (DKA).
The next step: A phase III clinical trial.
8. Leading Immune-Mediated Organizations Unite
Did you know that people with T1D are more likely to have a coexisting autoimmune disease? And someone with T1D is at a more than 3-fold increased risk of developing multiple sclerosis, as compared to someone who does not have type 1? And, if you have T1D and lupus, you are more likely to develop kidney complications, as opposed to people with just one disease.
That’s why Breakthrough T1D partnered with the Lupus Research Alliance and the National Multiple Sclerosis Society to advance the understanding of autoimmunity and to obtain more specific insights into commonalities and differences of immune pathways that govern these disease processes. The researchers will get a maximum of $450,000 for up to 2 years to generate the first tranche of results from their projects.
9. Eli Lilly Acquires Protomer Technologies
The Breakthrough T1D T1D Fund portfolio company Protomer Technologies has been acquired by Eli Lilly and Company. Protomer is developing glucose-sensing, or “smart,” insulin, which is designed to sense sugar levels in the blood and automatically activate as needed throughout the day. Smart insulin has the potential to revolutionize the treatment and quality of life of people with diabetes by improving both the effectiveness and safety of insulin therapy and significantly reducing the risks of both dangerous high and low blood-glucose episodes and life-threatening complications, leading to healthier, longer lives.
Breakthrough T1D has been an early supporter of smart insulin (also called glucose-responsive insulin, or GRI), and Breakthrough T1D and the T1D Fund have supported Protomer from before the company’s inception through its sale to Lilly. In 2013, Alborz Mahdavi, Ph.D., was a winner of the Breakthrough T1D Agnes Varis GRI Grand Challenge Prize. Subsequently, he founded Protomer, which was initially funded by Breakthrough T1D’s Industry Discovery and Development Program.
Following these successful grants from Breakthrough T1D, the T1D Fund made a minority equity investment in Protomer’s Seed round, alongside Eli Lilly, to advance the company’s highly promising glucose-sensing insulin program.
The successful sale of Protomer to Eli Lilly is a great example of how Breakthrough T1D research, the T1D Fund, and key diabetes industry players collaborate as a part of a larger ecosystem to help advance meaningful therapies for the T1D community.
10. Breakthrough T1D Centers of Excellence
A notable addition in our progress toward improving lives and curing T1D is the establishment of the Breakthrough T1D Centers of Excellence. There are now a total of five Breakthrough T1D Centers of Excellence: New England, Northern California, University of Michigan, Australia, and the University of British Columbia. These Centers of Excellence represent concentrated investments toward our goals by tackling a myriad of scientific questions that a single lab cannot undertake. By bringing together world-class, interdisciplinary research talents and resources at and across partner institutions, we are changing how we approach T1D research.
Movers, Shakers, T1D Changemakers
Your partnership is inseparable from these advances and many more. On behalf of our community, thank you for shaking things up—for more progress, more advancements, and more access—for everyone impacted by T1D.
Type 1 diabetes (T1D) and sleep, or lack thereof, are often tied together. Someone living with T1D must navigate a wide range of challenges that can impact sleep, and poor quality of sleep negatively affects your psychological and physical health.
In addition, one of the greatest fears of nighttime is low blood sugar (called hypoglycemia), which occurs when blood-sugar levels fall below 70 mg/dL. Symptoms can include shaking, an accelerated heart rate, and clammy skin. It’s possible to sleep through a hypoglycemic event, but you can wake up tired, confused, sweaty, and can feel lethargic or foggy.
But artificial pancreas systems are changing that.
Artificial pancreas systems (also called automated insulin delivery systems) have two interrelated functions: Monitoring the blood-sugar levels in your body (through a continuous glucose monitor) and automatically providing insulin to keep them in a healthy range (through an insulin pump), via a smart algorithm. By automating much of blood-sugar management, these systems can help improve diabetes-related outcomes and lighten the burden of T1D.
Even during sleep.
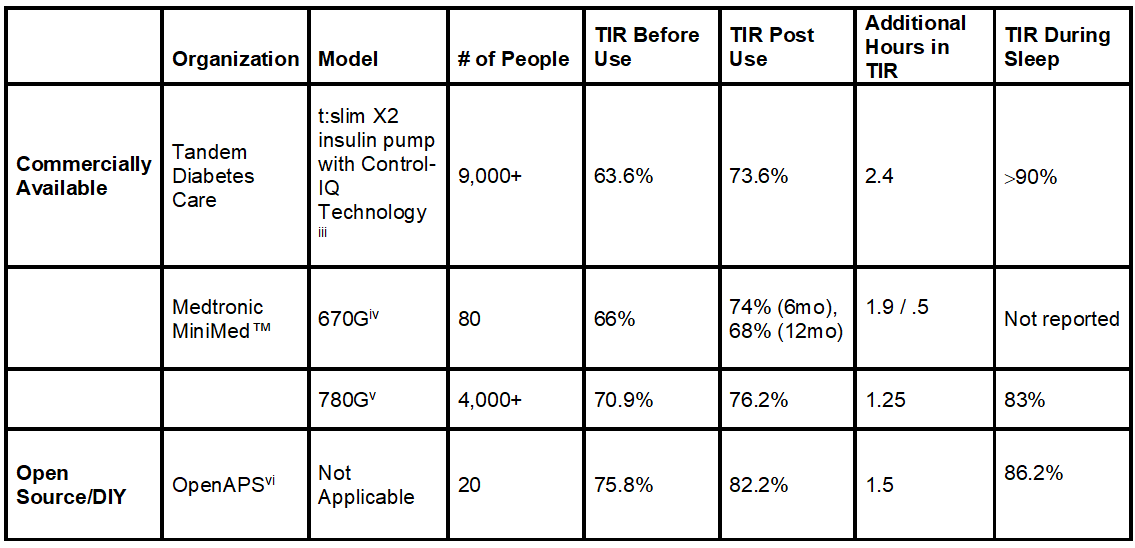
Time-in-Range and Sleep
Time-in-range (TIR) is a measurement that tells you what percentage of the day your blood sugars are in your goal range (typically 70-180 mg/dl). The American Diabetes Association recommends that people with diabetes spend 70% of their time in the target range, meaning you should aim for roughly 17 out of 24 hours each day to be in range. Unfortunately, less than half of the diabetes population meets this recommendation.[i]
But experts emphasize that even a 5% improvement in TIR—let’s say, going from 60% to 65%—is meaningful, as it translates to one more hour per day spent in-range.[ii]
Now, let’s look at some recent scientific findings in the table below.
Not only can the artificial pancreas systems improve time-in-range, but they can dramatically improve time-in-range during sleep.
Note: Left click on image to enlarge.
More time-in-range, even when you’re asleep. Now that’s research that can help you rest easy and more often than not, wake up on the right side of the bed.
Have a goodnight!
Watch Breakthrough T1D’s and Tandem’s Facebook Live event (video below) exploring AP systems and improvements in TIR and sleep.
Editor’s Note: This educational content is made possible with support from Tandem Diabetes Care. Breakthrough T1D produces this content to provide information to our supporters about their potential options for managing their T1D and not as an endorsement of products. Editorial control rests solely with Breakthrough T1D.
RX ONLY. The t:slim X2 insulin pump with Control-IQ technology is indicated for patients with type 1 diabetes, 6 years and older. BOXED WARNING: Control-IQ technology should not be used by people under age 6, or who use less than 10 units of insulin/day, or who weigh less than 55 lbs. For full safety information, visit tandemdiabetes.com/safetyinfo.
[i] Bergenstal RM, Hachmann-Nielsen E, Tarp J, Kvist K, Buse JB. 65-LB: Real-World Continuous Glucose Monitoring Data on Time-in-Range from a U.S. Population, 2015–2019. Diabetes Jun 2021, 70 (Supplement 1); doi: 10.2337/db21-65-LB
[iii] Breton MD, Kovatchev BP. One Year Real-World Use of the Control-IQ Advanced Hybrid Closed-Loop Technology. Diabetes Technol Ther. 2021; 23 (9): 601-608. doi:10.1089/dia.2021.0097
[iv] DuBose S, Bauza C, Verdejo A, Beck RW, Bergenstal RM, Sherr J. Real-world, Patient-Reported and Clinic Data from Individuals with Type 1 Diabetes using the MiniMed 670G Hybrid Closed Loop System. Diabetes Technol Ther. 2021; 10.1089/dia.2021.0176. doi:10.1089/dia.2021.0176
[vi] Lewis DM, Swain RS, Donner TW. Improvements in A1C and time-in-range in DIY closed-loop (Open-APS) users. Diabetes. 2018; 67: 352-OR. https://diabetes.diabetesjournals.org/content/67/Supplement_1/352-OR.abstract.
People living with type 1 diabetes (T1D) have numerous advanced technologies to choose from to better manage their T1D: Continuous glucose monitors (CGMs), insulin pumps, and artificial pancreas systems, which combine the two.
These technologies can make managing T1D easier and help facilitate better outcomes: Fewer experiences of low blood sugar (called hypoglycemia) or high blood sugars (called hyperglycemia), better sleep, and more time-in-range.
There is no such thing as too many options to choose from for T1D management—what works for one person may not be the best fit for another.
Now, people living with T1D who prefer multiple daily injections (MDI) of insulin have two high-tech T1D management options: An insulin smart pen or an insulin smart pen cap.
What are Insulin Smart Pens/Smart Pen Caps? How Do They Work?

These are innovative devices used for MDI insulin administration.
Medtronic’s InPen™ (a Bluetooth-enabled insulin pen) was first cleared by the FDA in 2016, but received FDA clearance for all ages* in 2020. Bigfoot Biomedical’s Bigfoot Unity™ (a Bluetooth-enabled insulin pen cap) received regulatory clearance for ages 12 and up this past year.
Like conventional insulin pens, insulin smart pens can be used to administer certain long- or rapid-acting insulin as needed while basal insulin pen caps can track long-acting insulin.
Unlike conventional insulin pens and insulin pen caps, InPen and Bigfoot Unity do a lot more than physically deliver insulin into the body—they are part of an intuitive diabetes management system.
InPen and Bigfoot Unity pair with corresponding smartphone apps through which a person with diabetes can keep track of insulin doses, blood-glucose levels (via data from a compatible CGM or blood-glucose meter), as well as receive reminders, alerts, and reports.
More specifically, insulin smart pens/caps can:
- Calculate insulin dosage based on current blood-glucose level, carbohydrate reading, meals, active insulin or insulin on board, and settings prescribed by a physician
- Deliver correct half-unit doses that are ½ units**
- Issue alerts to help prevent skipped or missed doses
- Crunch the numbers when you’re trying to calculate how to dose for a meal or correct high blood glucose
- Track the time and amount of every dose, and issue reminders for the next insulin dose
- Indicate whether insulin has expired or has been subjected to high temperatures so the cartridge can be replaced
- Provide diabetes data to your health care team at any time
- Sync with smart phones or watches, as well as popular diabetes data tracking platforms
Both InPen and Bigfoot Unity sync with CGMs that provide blood-glucose level data in real time; InPen with Medtronic’s Guardian™ Connect System or Dexcom and Bigfoot Unity with Abbott’s Freestyle Libre 2.
Both can also be used by people using blood-glucose meters. Automatic transmission of blood glucose data may require the meter to meet technological requirements.

This is crucial in that for the first time, people with T1D who do not wish to use any kind of wearable device, can finally reap some of the benefits of using more advanced T1D management technologies—and they have more than one choice. A huge step in bridging the T1D technology gap.
“One of Breakthrough T1D’s goals is to make sure that everyone has the diabetes management tools that work best for them,” said Associate Director of Research at Breakthrough T1D, Jonathan Rosen, Ph.D. “While we have driven forward the development of AP systems, which consist of a pump, CGM, and controller, and have demonstrated benefits in improving outcomes and reducing disease burden, we appreciate that not everyone wants to wear a pump and are thrilled to see the progress in smart pens like Medtronic’s InPen and Bigfoot Biomedical’s Unity. Smart pens can aid with many aspects of T1D care, including helping users determine when to take insulin and what the dose should be, which helps relieve the daily burden of T1D.”
“I am OBSESSED with My InPen”

Mattie Fisher is a wife and mother of two from Titusville, Florida. This marketing manager for a transportation firm is a plant-based lifestyle devotee who loves to spend time with her family outdoors—mostly engaging in water sports.
Diagnosed at age 12, Mattie has been living with T1D for 20 years. She can’t say enough good things about her InPen.
“I like to say I am OBSESSED—in a healthy way—with my InPen,” she said.
When she was first diagnosed, Mattie used vials of long-lasting and rapid-acting insulin, along with syringes and lots of finger sticks. When she was ready to enter high school, she decided to try an insulin pump.
Turns out, it wasn’t the right fit for her.
“I did not like being connected to something all the time. Sleeping was uncomfortable, it would snag on different things,” she said.
She, her parents, and her heath care team decided to switch to insulin pens.
“I have been on Humalog®1 pens and my long-lasting pens since age 16,” Mattie said. “The pens really seemed to suit my lifestyle better, but I did not get any of the perks that the insulin pump offered—having to manually do all the adding and subtracting for all meal boluses and corrections for any high blood sugars was not my strong suit.”
Related content: “You’re a Mathematician! No, I just have type 1 diabetes”
She said this often led to her simply using a number she was comfortable with for meals or corrections.
“And that led to guessing, which is not a good idea,” she said.
Insta Attraction
All of that changed in March 2021.
“I came across another person on Instagram who is living with type 1 who was using the InPen,” Mattie said. “I had no clue what the InPen was and messaged her with a few questions. After a couple messages back and forth, I knew I had to find out more.”
She called Medtronic for more information and was immediately taken with InPen.
“I love that it does much of the work for me, as far as calculating how many units [of insulin] I need for the meal I am about to eat,” Mattie said. “I also enter my blood glucose and the amount of carbs into the InPen App—and BAM!—it does the rest. Lastly, it also calculates my correction dosage, if needed. And it automatically adjusts if I still have insulin on board [active in my body].”
Mattie uses the InPen with Medtronic’s Guardian Connect CGM. And while she raves about how much easier her T1D management is now, that is not the only big benefit.
“The InPen tells me how much insulin I have used and keeps track of how much insulin I still have in my system,” she said. “I am having fewer instances of low blood sugars now and my confidence in how I am managing my diabetes has improved greatly—almost immediately from using the InPen. I am happy with how my diabetes is being managed and I know my doctor is as well.”
Soon to Come: Even More Choices
Reportedly, other companies are working on products similar to InPen and Bigfoot Unity.
These will provide more people who choose MDI even more new technology choices to help ease the burden of managing their T1D, as well as potentially improve how they manage their T1D.
Mattie is living proof that finding the “just right” technology can make all the difference, and she’s far from the only person singing the praises of the InPen.
Editor’s note:
*<7 requires adult supervision
**InPen only
-
Humalog ® is a registered trademark of Eli Lilly and Company
Oh. My. Gosh. I’m writing this blog, for Mathematics and Statistics Awareness Month, and I couldn’t believe what people with type 1 diabetes (T1D) have to do, in mathematics, to reach the right blood-sugar levels. It’s insane! It’s not an overstatement to say that, to effectively manage T1D, one needs to be a mathematician.
Keep in mind, this is all hypothetical. If you have T1D, talk to your doctor/endocrinologist if you have any questions about anything in this post.
Total Daily Insulin Requirement
Let’s take a seemingly simple mathematical equation: Total daily insulin requirement (in units of insulin).
You take your weight and divide it by 4. If you weigh 160 pounds, then:
160 ÷ 4 = 40 units of insulin per day.
Easy, right? No.
Because it might be too much insulin if you are newly diagnosed and still making a lot of insulin on your own or you are sensitive to insulin; or it might be too little if you are very resistant to insulin; or, if you eat more, there’s more insulin; if you eat less, it’s less insulin. (This is a general formula, so, as always, talk to your doctor/endocrinologist about the best insulin dose for you.)
Basal Insulin Dose
Next, you need to establish the basal (also called background) insulin dose, which is generally constant from day to day. A couple of things to know about insulin:
- Approximately 40%-50% of the total daily insulin dose is when it’s NOT mealtime (basal).
- The other 50%-60% is for carbohydrate coverage (mealtime) and high blood sugar correction (bolus).
Let’s say, again, you weigh 160, and your totally daily insulin dose is 40 units (160 ÷ 4 = 40 units).
Your basal dose is 40%-50% of your total daily insulin dose. Let’s say we make it 50%, so it’s:
50% of 40 units = 20 units (of either long acting insulin or intermediate insulin).
Bolus (Carbohydrate Coverage) Insulin Dose
Okay, you’ve gotten this far. But what about mealtimes? Now you have to remember—it can be calculated using the “Rule of 500.”
You take 500, and divide it by your total daily insulin dose (40 units) to get your carbohydrate coverage ratio:
500 ÷ 40 = 12 grams of carbohydrate per 1 unit of insulin.
Easy, right? Think again.
This assumes that you have a constant response to insulin throughout the day, but insulin-to-carbohydrate ratio varies. Someone who is resistant in the morning, but sensitive at mid-day, will need to adjust the insulin-to-carbohydrate ratio at different mealtimes. The insulin-to-carbohydrate ratio might be breakfast 1:8 grams, lunch 1:15 grams, and dinner 1:12 grams.
Carbohydrate Coverage at a Meal
1 unit of insulin = 12 grams of carbohydrate.
Let’s say you’re going to have a turkey sandwich for lunch. That’s 36 grams of carbohydrate.
36 grams ÷ 12 grams = 3 units of insulin.
You’ll need 3 units of short or rapid acting insulin to cover the carbohydrate.
But…
High Blood Sugar Correction Dose (Correction Factor)
High blood sugar correction is defined as how much 1 unit of rapid acting insulin will reduce the blood sugar so many mg/dL. This time, you have to remember the number 1800.
1800 ÷ Total Daily Insulin Dose (40 units) = 1 unit of insulin will reduce the blood-sugar level by 45 mg/dL.
Next, you have to calculate the high blood sugar correction dose.
(Actual blood sugar – target blood sugar) ÷ correction factor = high blood sugar correction dose.
Let’s say your actual blood sugar, before lunch, is 235 mg/dL, and your target is 100 mg/dL.
So 235 mg/dL – 100 mg/dL = 135 mg/dL, then we divide it by the correction factor (45), and we get 3 units of rapid acting insulin, to “correct” the blood sugar down to a target of 100 mg/dL.
So…
Total Mealtime Dose
To get the total mealtime insulin dose, add the carbohydrate coverage dose together with the high blood sugar correction dose, so:
3 units of insulin + 3 units of insulin = 6 units of rapid acting insulin for your lunch.
And this was just lunch!
There’s nothing bad you can say about John J. McDonough. He was a self-made man. He served on Breakthrough T1D’s International Board of Directors and was Chairman in 1999 to 2000. He and his wife, Marilyn, founded the BETA Society, for people who name Breakthrough T1D in their estate plans. He was a poet. Well, no, he wasn’t…but that didn’t stop him from trying. And, he had “the ability to laugh in the face of some pretty awful things.”
This was “one of his best qualities,” said Allison, his eldest daughter.
He passed away on February 16 at the age of 84, from complications of type 1 diabetes (T1D).
He was a champion. A philanthropist. And, above all, a friend. He will be sorely missed.
Early Years
John was age 6 when he was diagnosed with T1D, in 1943. This was well before insulin pumps, continuous glucose monitors, and engineered insulin were around. John was told he would likely die by age 15. But, against all odds, John thrived.
He met Marilyn when he was 16, she 15. He graduated high school and attended the University of Notre Dame. John loved working as a campus photographer. His favorite gigs were photographing Louis Armstrong and his band, and President Eisenhower. John and Marilyn married before he graduated with honors and entered the business world as an accountant.
They began having children right away, and would go on to raise 5 McDonoughs (and 1 son-in-law, 2 daughters-in-law, 8 grandchildren, 3 granddaughters-in-law, and 3 great-grandchildren!).
Successful and Significant Life
John’s determination to control the disease is what helped him defy the odds. He had a successful career in business and finance spanning more than 50 years and several industries: manufacturing, marketing, medical and dental products, and telecommunications.
But John wanted significance, too. When he received Notre Dame’s Sorin Award, which is presented to a graduate who has rendered distinguished service to the university and community, he said: “Success is important because it makes education, care of family, and a reasonable lifestyle possible. Significance is about making your life count. They can be simultaneous or sequential, but without significance, one’s life cannot be a success.”
 It was the diagnosis of his daughter, Allison, with type 1 in 1983 that led John to take a driving interest in moving T1D research forward.
It was the diagnosis of his daughter, Allison, with type 1 in 1983 that led John to take a driving interest in moving T1D research forward.
In addition to being a member of Breakthrough T1D’s Board and founding chairman of the BETA Society, he served on many special committees for both Breakthrough T1D International and Breakthrough T1D’s Illinois Chapter. John and Allison testified before Congress at Breakthrough T1D’s Children’s Congress in Washington, D.C. He received the Person of the Year Award from both the Breakthrough T1D Southern Florida Chapter and the Breakthrough T1D Illinois Chapter. He’s made significant gifts to help fund research for the past 25 years.
 “We are much farther down the path of curing type 1 diabetes because of John’s efforts,” said Joe Lacher, now chairman of the Breakthrough T1D International Board of Directors. “He was incredibly proud of the innovations—like continuous glucose monitors and the artificial pancreas—that benefited John, Allison, and the type 1 diabetes community. It is because of his efforts that people with the disease are living longer and healthier lives and curing type 1 is within reach.”
“We are much farther down the path of curing type 1 diabetes because of John’s efforts,” said Joe Lacher, now chairman of the Breakthrough T1D International Board of Directors. “He was incredibly proud of the innovations—like continuous glucose monitors and the artificial pancreas—that benefited John, Allison, and the type 1 diabetes community. It is because of his efforts that people with the disease are living longer and healthier lives and curing type 1 is within reach.”
Love of Life, and Silly Jokes
You know how I said that the remarkable thing about John was his humor? Even in the face of diabetes complications, even in the face of losing his leg, he always had that.
“After Dad lost his leg, we went together to get his first prosthetic leg,” recalled Allison. “Sitting in the waiting room, I was very surprised when all this grief welled up inside of me and I just started to weep. There I was, wailing away, and I could tell Dad was very upset because I was so upset. He said, ‘Hon, this amputation is really the best thing that could have happened.’ I just wasn’t in the mood to be spiritually advanced and was about to tell him that I loved his attitude, but I just couldn’t be positive right then. Then he continued, ‘I only worry about athlete’s foot half as much as I used to.’”
“I wasn’t expecting a joke,” said Allison. “I was stunned for a moment, but then we both started laughing through our tears. If others in the waiting room thought we were loons, I didn’t care. Once again, Dad showed his amazing ability to not let his diabetes dampen his spirit.”
Let’s hope that, someday, T1D won’t let anyone dampen one’s spirit again—because we have found cures for the disease. Until that day comes, we will fund research that will get us there.
We also wanted to share a recent video, featuring John, and his amazing spirit. John, you will be missed.
Accommodations. You might not know that it’s an important word, for anyone who has a disability. And, if you’re a person with type 1 diabetes (T1D), you have a qualified disability, and can request reasonable accommodations to help you go to school or work.
But what about COVID-19 and T1D? To help you traverse the school and employment rights, Breakthrough T1D had a Facebook Live event with Daniel Phelan, Esq., an attorney who’s an associate at Duval and Stachenfeld LLP and chairman and CEO of the Type 1 Action Foundation. He was diagnosed with T1D when he was in seventh grade, just a few weeks after his 13th birthday.
You’ll learn:
|
General |
|
|
Children
|
|
|
Employment Rights for Adults with T1D who Work in Schools
|
|
|
Workplace Employment Rights for Adults with T1D
|
|
Check out the full, recorded Facebook Live event below.
In type 1 diabetes (T1D), receiving transplanted donor beta cells restores blood-sugar control and significantly improves people’s quality of life. The current approach, however, is available to a limited number of people and these individuals need to take immunosuppressive drugs for the rest of their lives.
Our ambition at Breakthrough T1D is to bring beta cell replacement therapies to the broader T1D community with renewable cell sources and without the need for chronic immune suppressing drugs.
We’ve already solved a key challenge: After more than a decade of research, steered by Breakthrough T1D, Douglas A. Melton, Ph.D., and colleagues have solved the major obstacle of generating unlimited numbers of beta cells using stem cells, in 2014. Now, Dr. Melton, the principal investigator on the new New England Center of Excellence, will focus on the challenge of protecting these cells from rejection by the immune system that occur following transplantation.
Dr. Melton, in partnership with Breakthrough T1D research leadership, has assembled a multidisciplinary team that includes key diabetes investigators involved in cellular biology and immunology research, from five institutions: Harvard University, University of Massachusetts Medical School, Joslin Diabetes Center, The Jackson Laboratory, and Dana-Farber Cancer Institute.
They are applying their talents toward one of the most exciting, emerging fields: Gene modification, or gene editing. They are using gene modification technology to create beta cells that can withstand immune attacks and utilizing these engineered cells in the development of T1D therapies.
“I am truly blown away by the research at the Breakthrough T1D Center of Excellence in New England,” says John Cammett, co-founder of Realterm Global, president/CEO and co-founding partner of Aeroterm, and a seed funder of the Breakthrough T1D Center of Excellence in New England. “My own desire to find a cure is inspired by my mom living with type 1 diabetes for almost 60 years.
“We have supported several of the researchers at this Breakthrough T1D Center of Excellence individually before,” he describes. “But the Breakthrough T1D Center of Excellence in New England excites me and gives me tremendous optimism because it brings together the brightest minds, along with the entire Breakthrough T1D ecosystem, with a laser-sharp focus on developing cures. I encourage others to provide financial support, because it’s collectively between these researchers’ intellect and our financial resources that we will cure type 1 diabetes.”
“The Breakthrough T1D Center of Excellence in New England excites me and gives me tremendous optimism because it brings together the brightest minds, along with the entire Breakthrough T1D ecosystem, with a laser-sharp focus on developing cures.”
—John Cammett
Seed Funder, Breakthrough T1D Center of Excellence in New England
This is the third Center of Excellence launched by Breakthrough T1D, the first one in Northern California with Stanford University and the University of California, San Francisco, and the second at the University of Michigan.
The Northern California Center will focus on the interaction of immune cells with insulin-producing beta cells; generating islets and immune cells from stem cells for next-generation therapies; and developing ways to transplant insulin-producing cells into people with T1D without requiring immunosuppression.
The Michigan site will focus on undertaking a comprehensive definition of T1D metabolism, with the goal of preventing or treating short- and long-term complications—like low or high blood-sugar levels; eye, kidney, heart, and nerve diseases; and brain impairment and psychosocial stress—that arise from the disease, and aiming to identify drug targets that can be used in beta cell replacement therapy.
To learn more, read our press release.
The researchers involved in the Breakthrough T1D Center of Excellence in New England:
Harvard University
Douglas A. Melton, Ph.D. (principal investigator)
University of Massachusetts Medical School
Michael A. Brehm, Ph.D. (co-principal investigator)
Dale L. Greiner, Ph.D.
David M. Harlan, M.D.
Sally C. Kent, Ph.D.
René Maehr, Ph.D.
Joslin Diabetes Center
Jason Gaglia, M.D. (co-principal investigator)
Stephan Kissler, Ph.D. (co-principal investigator)
Peng Yi, Ph.D.
The Jackson Laboratory
Lenny Shultz, Ph.D.
Dana-Farber Cancer Institute
Judith Agudo, Ph.D.
After a thorough national search, we are pleased to announce that Timothy Doyle will join Breakthrough T1D as President and Chief Operating Officer (COO), effective February 1, 2021.
Tim will report to Aaron Kowalski, Ph.D., who will continue to serve as Breakthrough T1D’s Chief Executive Officer.
He will serve as a critical strategic partner to Aaron, leading organizational operations to broaden Breakthrough T1D’s reach and impact, working with Breakthrough T1D’s executive team and International Board of Directors.
Tim’s appointment will enable Aaron to focus on overall strategy and take a more active, external-facing role driving growth and accelerating mission as the face and voice of Breakthrough T1D.
Tim is a highly experienced and well-regarded leader with a track record of success developing and implementing strategic plans and management strategies in higher education and research institutions. He comes to Breakthrough T1D from the Carnegie Institution for Science, where he served as COO.
Prior to that, he was Associate Dean for Finance and Chief Financial Officer (CFO) for Harvard’s School of Engineering & Applied Sciences. Tim earned a Master of Business Administration degree from Suffolk University and served 13 years in the United States Army. His ability to build high-performing teams and collaborate across complex organizations will be a tremendous asset to Breakthrough T1D.
“As we begin to see light at the end of the COVD-19 tunnel and come into 2021 prepared to propel Breakthrough T1D to an even higher level of impact, we look forward to the value Tim brings to Breakthrough T1D,” Kowalski said. “I am confident that Breakthrough T1D will greatly benefit from Tim’s expertise as we focus on accelerating our mission through significant growth in the years to come and meaningfully improving lives during every stage of the type 1 diabetes journey.”
Tim’s passion for Breakthrough T1D’s mission is fueled by family members, colleagues, and friends who live with T1D.
Please join us in welcoming Tim as he joins Breakthrough T1D and works to advance the mission we all share.