Most people with type 1 diabetes (T1D) and their caregivers are well aware of the dangers of hypoglycemia (low blood sugar)—especially should it become severe, which can result in seizures or unconsciousness.
Many families don’t talk about the reality of a severe hypoglycemic episode, much less plan for it as the topic can lead to anxiety for all involved
This increased anxiety is so common that there’s even a term for it: Fear of Hypoglycemia (FoH).
According to research published in the June 2019 supplement issue of the journal of the American Diabetes Association, FoH is associated with substandard diabetes management and reduced health outcomes.
Breakthrough T1D and our partners at Beyond Type 1 want T1D families to know that it doesn’t have to be this way.
With proper planning, preparation and provisions to quickly treat severe hypoglycemia, you can overcome FoH and handle emergencies with confidence.
Why now?
The coronavirus pandemic has upended the lives of most Americans. For many families, remote learning and working has created a new, stress-filled normal in which caregivers are always on and stretched thin. New routines present additional challenges. In the process, steps to prevent hypoglycemia may accidentally be skipped, increasing the chances for severe hypoglycemia.
But quarantine life also offers the opportunity to talk about and plan for important issues like severe hypoglycemia.
How should you prepare?
For starters, each member of a T1D family should be aware of the different causes and symptoms of hypoglycemia, as well as what mild/moderate hypoglycemia looks like vs. severe hypoglycemia.
A type 1 diabetes family should always have glucagon on hand to be prepared to handle a severe hypoglycemia emergency—which can be associated with seizures and unconsciousness. Each family member should know where the glucagon it is kept, how to administer it and the steps to take after administering it:
- Turning the person on their side. This is important because glucagon may cause nausea and vomiting and turning the person on their side will prevent them from choking if they do vomit.
- Calling 911 and staying by the person’s side until emergency responders arrive are taking care of the person.
Glucagon comes in different forms:
- Baqsimi—a nasal spray (Lilly)
- GlucaGen (glucagon) for Injection (Novo Nordisk)
- GvokePFS (glucagon injection) Pre-Filled Syringe (Xeris)
Families should check their glucagon at the same time each year to make sure it has not expired. This includes glucagon that may be kept in the car or is part of a travel emergency kit.
Don’t forget communication
Because severe hypoglycemia can limit someone’s ability to communicate clearly—even if they  remain conscious—T1D families may want to establish some kind of signal that their loved one with T1D can make to let others know they need help right away.
remain conscious—T1D families may want to establish some kind of signal that their loved one with T1D can make to let others know they need help right away.
This could be as simple as a specific word, blinking or stomping one’s feet two or three times. Whatever the signal is, it needs to be something the person with T1D feels they could likely do even if they are feeling very low, and something everyone in the family will remember.
You should work together to test out the signal when your loved one with type 1 diabetes has a stable blood sugar level and is feeling normal. That way, you will see if the signal makes sense for everyone involved. Don’t be afraid to have “practice drills” to make sure everyone knows how to respond.
You may also want to map out responses in different scenarios. For instance, a plan for what to do at home as well as a plan for what to do when you are away from home (does everyone know where the glucagon is kept in the car, on vacation, at a relative’s house, etc.?).
Don’t hesitate to ask for help
People can be reluctant to ask for help—even when they most need it. Sometimes, they may not even be aware that they need help.
Remind your loved one with type 1 diabetes that they should never hesitate to ask for help and emphasize to other family members that they should be ready to help at a moment’s notice.
Fear of Hypoglycemia is a real thing and quite common. But it can be overcome.
For additional advice about severe hypoglycemia emergency plans, T1D families should consult with their healthcare providers, and ask about which glucagon option is best for them.
Editor’s Note: This educational content is made possible with support from Lilly Diabetes and BD. Breakthrough T1D produces this content to provide information to our supporters about their options for managing their T1D and not as an endorsement of products. Editorial control rests solely with Breakthrough T1D.
On June 15, 2020, Abbott announced that the FDA has authorized the FreeStyle Libre 2 System. The Libre 2 gained its CE Mark clearance in Europe late 2018 and is now authorized for adults and children 4 and up in the United States. The FreeStyle Libre 2 is a 14 day continuous glucose monitor (CGM) system that transmits data every minute and now includes customizable high and low alerts without the need to scan the device. The Abbott announcement says it will be “a third of the cost of other CGMs.”
Also notable with this clearance is the FDA’s decision to permit the marketing of the FreeStyle Libre 2 as an integrated CGM (iCGM). This allows the CGM to be part of an interoperable system and work with other medical devices like insulin pumps, blood glucose monitors and other devices. This makes the FreeStyle Libre 2 only the second CGM on the market to receive this designation.
This also marks the first time a FreeStyle Libre CGM has been approved for pediatrics in the United States, widening the scope of potential users.
According to Abbott, the Libre 2 adds easy-to-use, customizable alarms for high and low glucose levels using Bluetooth. Users can also receive notifications should the sensor and reader fail to link up to ensure that a dropped connection does not go unnoticed. Alerts come in the form of sounds or vibrations, according to preference. Libre 2 users can still scan their sensor as often as desired to see current blood glucose levels, trends and patterns and a graph of the last eight hours.
“Another iCGM provides people with diabetes more choice to improve diabetes outcomes,” says Campbell Hutton, Vice President, Regulatory and Health Policy, Advocacy at Breakthrough T1D, “and also will facilitate diabetes device interoperability.”
Content from this article was sourced from Beyond Type 1.
Scientists have uncovered a potential breakthrough in developing a faster acting insulin in an unusual place: snails. Now, Breakthrough T1D-funded researchers are using the molecular features of the snail insulin to create ultra-rapid insulins for people with type 1 diabetes (T1D) and have published their findings in .
As discussed in last year’s blog, certain sea snails shoot insulin at their prey, sending them into hypoglycemic shock and allowing the snails to eat them. This immediately piqued the interest of T1D scientists, and catalyzed research into better understanding how this insulin works, with the potential to transform the treatment of T1D.
The human and rapid-acting analog insulins available today leave much to be desired. They are slow to lower blood sugar and remain in the bloodstream for hours after administration. Faster acting, or ultra-rapid insulins, would help people with T1D better manage their disease. They could correct high blood sugars more quickly, allow for more flexibility at mealtimes, prevent hypoglycemia caused by insulin lingering too long in the body, and crucially, help to “close the loop” in artificial pancreas technology, i.e. create an artificial pancreas system where the user doesn’t have to manually enter any insulin doses (for example, at mealtime).
This paper, whose authors include lead investigator Xiaochun Xiong, Ph.D., a Breakthrough T1D postdoctoral fellow; Breakthrough T1D-funded senior author Danny Chou, Ph.D.; and previously Breakthrough T1D-funded collaborator Helena Safavi-Hemami, Ph.D., details their approach. These scientists identify the molecular characteristics of the snail insulin that make it work quickly and potently and incorporate them into human insulin. This creates an insulin that is part human and part snail that will serve as a basis for new ultra-rapid insulins.
In order for people with T1D to achieve better outcomes, insulin needs to work faster. Breakthrough T1D is excited by the findings in this paper, and looks forward to continuing to support this science to achieve novel, innovative insulins.
Read the paper here and learn more about Breakthrough T1D’s research into glucose control here.
During a recent Breakthrough T1D Facebook Live event, a panel from Medtronic Diabetes discussed Medtronic’s efforts to support the T1D community during the coronavirus disease (COVID-19) pandemic.
Members of the panel included Executive Vice President and President of Medtronic Diabetes Sean Salmon, as well as other leaders from Medtronic. Breakthrough T1D Vice President of Corporate Development Joe Watterson and Senior Director of Marketing for Medtronic Diabetes Charles Cush delivered introductory remarks.
The panelists addressed a variety of topics, including:
-
Medtronic’s COVID-19 response, including how they have been listening to and working with the T1D community and health care professionals (presented by Salmon)
-
Medtronic Assurance Program (presented by Vice President of Health Economics, Reimbursement & Government Affairs, Medtronic Diabetes, Jeff Farkas)
-
Optimizing Remote Telemedicine Appointments (presented by Chief Medical Officer, Medtronic Diabetes, Robert Vigersky, M.D.)
-
Supporting People Living with T1D in a Virtual World (presented by National Clinical Director, Medtronic Diabetes, Kim Larson)
Watch the full, recorded livestream:
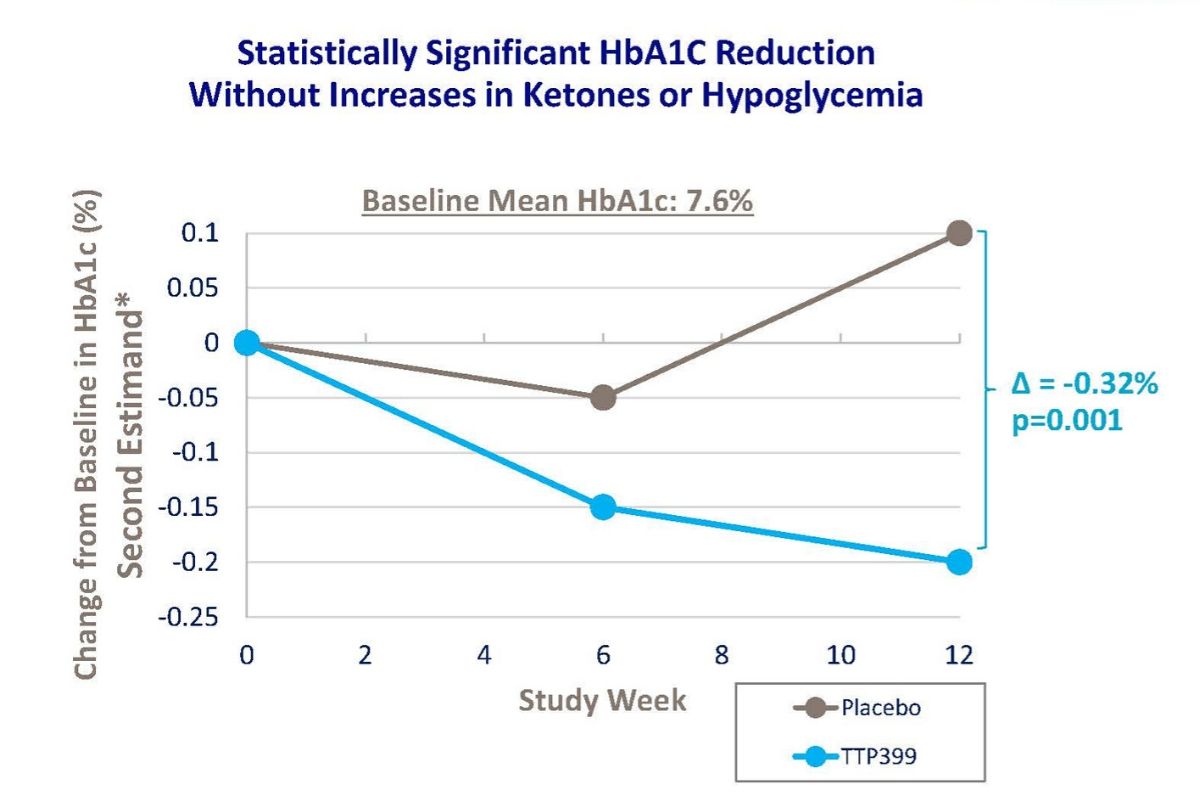
Nearly 80 percent of people with type 1 diabetes (T1D) fail to meet HbA1c goals defined by the American Diabetes Association. Despite more widely adopted diabetes technology and an increase in use, there is no improvement in clinical outcomes. This tells us that the current use of insulin alone is not enough, but vTv Therapeutics may have an answer.
In a Breakthrough T1D-funded clinical trial, they tested the safety and efficacy of TTP399, an oral therapy to be used in conjunction with insulin, in 85 people with T1D. The study successfully achieved its primary objective by demonstrating statistically-significant improvements in HbA1c, compared to placebo, at week 12.
The daily time-in-range was improved by approximately two hours in people treated with TTP399 relative to placebo. TTP399 was well tolerated, and importantly, there were no reports of diabetic ketoacidosis—a complication of T1D—reported in either group. There was no incidence of severe low blood sugar in the treated group and only one incident in the placebo group. People taking TTP399 experienced fewer symptomatic low blood sugar episodes: two subjects taking TTP399 reported at least one event compared to eight subjects in the placebo group (no TTP399).
TTP399 is a glucokinase (or GK) activator. GK acts as a key regulator of sugar levels in the body. If blood glucose levels are deemed too high, activation of GK in the liver has been shown to increase glucose utilization, which in turn lowers glucose levels in the blood.
vTv Therapeutics joined forces with Breakthrough T1D in 2017, to test TTP399 in people with T1D. The positive topline results from this phase II clinical trial follow the positive results obtained in the previous smaller clinical study reported by Breakthrough T1D in June 2019.
The next step: A phase III towards the future path of a registration trial. Stay tuned.
PhysioLogic Devices, a Breakthrough T1D-funded company that is dedicated to transforming the treatment of type 1 diabetes (T1D), wants to develop the ThinPump™—a fully implantable automated insulin delivery system—in the next four years. We think it’s not only possible, but doable. PhysioLogic has two things going for it:
- Vast knowledge of and experience with the implanted insulin delivery system, which delivers insulin directly into the abdominal space (called the intraperitoneal route). This allows the body to rapidly absorb insulin through the liver, which plays a central role in blood glucose control, and quickly clear insulin from the blood
- The funding of two world-renowned institutions: Breakthrough T1D and the company W. L. Gore & Associates (Gore), which invested in PhysioLogic last month
Gore is well known for developing GORE-TEX Products, the waterproof and breathable laminates used in outerwear. But that’s not all the company creates. It also makes air filters, headlight vents, heart stents and more. Gore was established more than 60 years ago, and generates annual revenues of $3.7 billion. Recently, Gore has started investing in the development of novel diabetes management technologies.
The standard of care for people with T1D involves insulin injections under the skin, known as the subcutaneous route. There are several known problems with this route of insulin delivery. First, the absorption of insulin from under the skin is slow, causing spikes in blood sugar after a meal. Second, the absorption of insulin from under the skin may continue for several hours after an injection, long after a meal has been fully absorbed. This causes blood sugar to fall to low levels hours after a meal. Slow insulin absorption and slow insulin clearance together cause blood glucose to alternately go high and low, outside of the normal range, in a roller-coaster manner that is well-known to people with T1D. It also makes it challenging to manage blood sugar levels during and after exercise.
But both of these problems can be corrected by delivering insulin into the abdominal space—via the intraperitoneal route. This route allows insulin to be processed in a manner that more closely resembles what happens in the body of someone without diabetes and the advantages of intraperitoneal insulin delivery over subcutaneous insulin delivery have been established in multiple clinical studies.
PhysioLogic’s mission is to create a fully implantable automated insulin delivery system—the ThinPump™—using intraperitoneal insulin delivery and an accurate and reliable glucose sensor, which could enable fully automated blood sugar control with no required user input. In addition, users would no longer have to worry about on-body devices or managing infusion set insertion sites. It’s a lofty goal, to be sure, but PhysioLogic is aggressively working towards starting the first-in-human clinical studies in less than four years.
In Europe, a similar product, Medtronic MiniMed™ Insulin Pump, has been used since the early 1990s. But translation of the MiniMed™model intraperitoneal insulin pump into the wide population has been small, due to several factors. The MiniMed™ model shows a bulge in the abdomen after transplantation, making it less attractive. And, manufacturing costs were too high. The ThinPump™ is predicted to be half the thickness of the MiniMed™ model, making it inconspicuous and significantly reducing manufacturing costs. Breakthrough T1D’s investment is bringing more dollars to T1D, and we celebrate this new line of funding for PhysioLogic Devices, which may lead to commercialization of this innovative technology. You can learn more about PhysioLogic Devices by visiting their website: PhysiologicDevices.com
Earlier this week, Beta Bionics announced that the Food and Drug Administration (FDA) has granted breakthrough device designation to the company’s iLet Bionic Pancreas System, a wearable pocket-sized device used for blood sugar control in people with diabetes. The device can be adjusted to work as an insulin-only, glucagon-only or bi-hormonal artificial pancreas using both insulin and glucagon.
Breakthrough T1D funded Ed Damiano, Ph.D., CEO of Beta Bionics, for his early research testing the safety and efficacy of a novel closed-loop system that incorporated the use of glucagon in addition to insulin. The results of this work helped to inform the development of the iLet bionic pancreas, which includes a configuration with a dual-chamber pump containing insulin in one chamber and glucagon in the other.
How is the iLet bionic pancreas system different than other artificial pancreas systems? Well, unlike most closed-loop systems on the market and in development, which use only insulin to normalize blood sugar levels, iLet has the ability to pump two hormones: insulin and glucagon. Glucagon, a key hormone whose response is impaired in type 1 diabetes (T1D), can help raise blood sugar levels when they become too low. This dual-hormone approach may enable the device to achieve tighter blood sugar control while minimizing low blood sugar, called hypoglycemia.
The Breakthrough Device Program is an FDA platform for more effective treatments of serious diseases, like T1D, and enables timely access to these devices by speeding their development, assessment, and review. Other companies that have received the FDA Breakthrough Device designation for T1D technologies include Breakthrough T1D partners EOFlow, Medtronic and Bigfoot.
What’s more, the iLet is designed to have the users enter only their weight for the iLet to initialize therapy. Immediately thereafter, the iLet begins controlling blood sugar levels automatically, without requiring the user to count carbohydrates, set insulin delivery rates, or deliver additional insulin for meals or corrections.
The iLet, if results are positive, would be a welcomed additional approach to help people manage their T1D.
The Food and Drug Administration (FDA) today authorized an algorithm that enables the second artificial pancreas system: The Control-IQ™ advanced hybrid closed loop technology.
For now, the algorithm can be used with Tandem’s t:slim X2™ insulin pump and Dexcom’s G6 continuous glucose monitor (CGM). Though this is not the first algorithm approved for an artificial pancreas system, it is the first algorithm authorized as an interoperable automated glycemic controller, which means the algorithm could be a component of any open protocol, or interoperable, artificial pancreas system. Formerly, there was only one FDA approved artificial pancreas system on the market: The Medtronic 670G, approved in 2016.
Enabling open systems is a priority for Breakthrough T1D—meaning that insulin pump, a CGM and an algorithm can “talk” to each other, regardless of the manufacturer. This gives the T1D community more choice and flexibility to select the technology that works best for them.
Breakthrough T1D has been a leader in the development of artificial pancreas systems for 15 years, since starting the Breakthrough T1D Artificial Pancreas Project in 2005 and the Breakthrough T1D Artificial Pancreas Consortium, which have dramatically accelerated progress by bringing academic researchers, government agencies, industry and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with increasingly advanced versions of the device. Manufacturers embraced the roadmap to guide their own research and development programs.
- Breakthrough T1D has funded over $110 million to date in artificial pancreas research.
- As testing new artificial pancreas technology in people with T1D can be challenging, Breakthrough T1D partnered with the FDA to create a regulatory pathway for approval and commercialization of this technology, leading to the 2012 FDA guidance for artificial pancreas systems. Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system in 2016, the first approved system.
- Breakthrough T1D continues to advocate with private and government insurers for choice and affordability of all treatments and technology for people with T1D. In particular, Breakthrough T1D calls upon United Healthcare to lift its restrictions on insulin pump coverage, which currently prevents its members with T1D from accessing technology like that authorized by FDA today.
Data for the submission to FDA came from the International Diabetes Closed Loop Protocol-3 (iDCL) trial, a six-month closed loop study, funded by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) with funds from the Special Diabetes Program (SDP). The clinical trial, with 168 participants ages 14+, which was recently published in the New England Journal of Medicine, met all of its primary and secondary endpoints, including time-in-range and HbA1c, with no fingerpricks and no severe low blood sugar events—a dangerous event for people with T1D, which, if not caught early, can lead to seizures, coma or, in extreme cases, death.
This important development is also a testament to the importance of the Special Diabetes Program (SDP), which has been renewed by Congress numerous times thanks to leadership by Breakthrough T1D.
The Special Diabetes Program (SDP) is up for renewal, so if you haven’t done so already, please sign up to be an advocate, and encourage friends and family to do the same. You’ll receive timely updates and actions you can take to turn Type One into Type None.
This is the latest example of how Breakthrough T1D research and advocacy work together to make T1D management better and safer. This is a win for the T1D community, and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
Breakthrough T1D is funding work to build a better insulin infusion set; the current infusion sets can be uncomfortable and unreliable, and need to be changed every two to three days. One partner is Pacific Diabetes Technologies, who will test its all-in-one continuous glucose monitoring (CGM) sensor and infusion set in people with type 1 diabetes (T1D) in a clinical trial at St. Vincent’s Hospital in Melbourne, Australia, under David O’Neal, M.D. The device, it is hoped, will eliminate the need for pump users to stick themselves twice—once to insert their CGM sensors, and again to insert their infusion catheters—improving the performance of both functions and making insulin dosing more accurate and comfortable. By making CGM a feature on an infusion set, it will also cut the number of devices worn on the body from two to one. Future versions will also offer an option to work with needleless Smart Pens for those who prefer multiple daily injections (MDI).
“A single insertion infusion line combined with a glucose sensor that has the potential to last longer than current insulin delivery platforms will reduce the burden for people with diabetes who choose to wear pumps,” says David O’Neal, M.D. “We are looking forward to implementing the research project and learning from the results.”
This is part of our Industry Discovery and Development Partnerships, which enables Breakthrough T1D to tap into the intellectual and financial resources of private entities working in the T1D space to help move therapies to market more quickly. At the front end, early Breakthrough T1D investments in promising therapies can make them less risky for industry partners to pursue. In later stages, the partners’ expertise in pushing products through the development pipeline and the regulatory approval process speeds their delivery to the T1D community. The therapies and devices at the heart of these partnerships are a direct result of nearly 50 years of investment and agenda-setting on the part of Breakthrough T1D, the world’s leading charitable funder of type 1 diabetes research.
Partnerships like these allow Breakthrough T1D to leverage its resources, tapping into the expertise and assets of industry stakeholders to fuel innovation and develop better therapies to help people with T1D stay healthy while we work toward cures.
A protein, called glucokinase (or GK), acts as a key regulator of sugar levels in the body. If blood glucose levels are deemed too high, activation of GK in the liver causes the body to use more glucose, which in turn lowers glucose levels in the blood. vTv Therapeutics has developed a GK activator, called TTP399. The company joined forces with Breakthrough T1D in 2017, to test TTP399 in people with type 1 diabetes (T1D). The results of the first phase of it are out, presented at the 55th Annual Meeting of the European Association for the Study of Diabetes (EASD).
Nineteen people were recruited for the Breakthrough T1D clinical trial—eight to the group getting TTP399, and 11 to placebo. At 12 weeks, the TTP399 group had a significant and clinically meaningful reduction of 0.6 percent in their HbA1c, whereas those in the placebo group increased their HbA1c by 0.1 percent. At the same time, the group treated with TTP399 showed a trend of decreased insulin usage. There was no severe hypoglycemia and no DKA—both of which are complications of T1D—reported in either group.
These promising findings support advancing to phase II-part 2 to confirm the results in a larger and more diverse T1D population.