Partners in clinical trials
Clinical trials are key to bringing new treatments, therapies, and devices for type 1 diabetes (T1D) from the lab to the clinic. Pharmaceutical and biotech companies, which conduct research and help develop and manufacture new therapies, are a critical player in accelerating this process. Companies often partner with clinical researchers at medical centers and hospitals to test their new therapies through clinical trials. These collaborative partnerships are essential for new drugs to move through the pipeline and get to people with T1D.
Clinical trials terms you need to know
Sponsor
The trial sponsor is the person, organization or company that initiates, manages, and oversees a clinical trial, ensuring it is conducted ethically and safely.
Trial site
A trial site is the medical center, hospital, or clinic where clinical trial participants receive the study drug, treatment, or intervention and receive care throughout the duration of the trial.
Principal investigator
Within a particular trial site, the Principal Investigator (PI) is the lead researcher that oversees the trial and the research team. The PI ensures participant safety, supervises data collection and analysis, and maintains scientific and ethical integrity during the research process.
Spotlight on the CATT1 clinical trial
The CATT1 clinical trial is investigating whether cadisegliatin (TTP399), a glucokinase activator, can reduce hypoglycemic events (low blood sugars) in people with T1D when used alongside insulin therapy. Read on to learn more about the people making the CATT1 clinical trial happen, the science behind cadisegliatin, and how you can get involved.
The CATT1 clinical trial
Sponsor
vTv Therapeutics (a company with support from the T1D Fund: A Breakthrough T1D Venture)
Trial site spotlight
The University of North Carolina (UNC) Medical Center located in Chapel Hill, N.C.
Principal investigator spotlight
Klara Klein, M.D., Ph.D., Assistant Professor of Medicine and Director of the Endocrinology, Diabetes, and Obesity (EnDO) Clinical Research Unit (CRU) at the UNC School of Medicine, Division of Endocrinology and Metabolism
Clinical research team member spotlight
Alex Kass, MSN, MBA, RN, CDCES, Research Program Director for the EnDO CRU
Breakthrough T1D x vTv Therapeutics
Breakthrough T1D has a long-standing relationship with vTv Therapeutics. After partnering with Breakthrough T1D in 2017, vTv pivoted from type 2 diabetes to T1D. With Breakthrough T1D’s support, vTv launched a phase 2 clinical trial (SimpliciT1) investigating cadisegliatin as an adjunctive therapy for T1D, showing that it improved blood glucose control. Eager to keep the momentum going, the T1D Fund has supported vTv from early 2024 onwards, most recently participating in the company’s latest round of financing to continue to support the ongoing phase 3 trial for cadisegliatin in T1D.

“Breakthrough T1D has long been a supporter of adjunctive therapies, meaning medicines that can be taken alongside insulin to improve glucose control and other outcomes. Previous clinical trials have demonstrated the potential for cadisegliatin to reduce hypoglycemia and improve HbA1C in people with T1D, and we are excited to support the phase 3 program to advance this therapy toward the clinic where it can be used to address major unmet clinical needs in our community.”
Jonathan Rosen, Ph.D., Director of Research at Breakthrough T1D
Meet the team at UNC
Within the UNC Medical Center, the EnDO CRU is a hub for endocrinologists, clinical researchers, and other experts who work together to lead clinical studies at cutting edge facilities that help bring new transformative therapies to the people who need them most. Within the EnDO CRU, Dr. Klara Klein and Alex Kass are taking the lead on T1D clinical trials—a passion they both share for different reasons.
T1D has been on Dr. Klein’s mind since she was a little girl. “I grew up hearing about diabetes—my mom is an endocrinologist, and it was a frequent topic at the dinner table,” she said. After following in her mother’s footsteps and becoming an endocrinologist herself, Dr. Klein is shocked that insulin—which was discovered over 100 years ago—is still the gold standard for T1D management. In this sense, not much has changed since she was sitting at the dinner table with her parents.
This sentiment inspires her work. “I’ve always been struck by the daily challenges people with T1D face. Even with incredible advances in technology, managing T1D is a constant effort—every meal, every workout, every illness,” she explained. Her true passion is bringing new therapies to fruition that can ease the daily burden of living with T1D until everyone has access to cures. “Being part of research that could improve the lives of people living with diabetes in meaningful ways is both exciting and deeply rewarding.”
Dr. Klein is supported by a passionate and experienced team composed of five endocrinologist sub-investigators, a nurse coordinator, and a research assistant. She also works closely with Kass, who leads the operational side of many different trials within the EnDO CRU.
Kass is one of a few other staff on the team who is also living with T1D, giving him an inside perspective and inspiring his career to help people with diabetes live better lives. “Having type 1 diabetes myself has fueled my passion for both patient care and finding better treatment options,” he explained. “Together, our team’s mission is simple: to run high-quality studies that give people with diabetes access to promising therapies and make their day-to-day lives easier. This shared passion in our group inspires me!”
The team is working hard behind-the-scenes to recruit and care for participants of the CATT1 clinical trial, which is just one of the many studies they are running to help people with T1D.
Everything you need to know about the CATT1 clinical trial
The phase 3 CATT1 clinical trial is investigating whether cadisegliatin can reduce moderate to severe low blood sugar (hypoglycemic) events when used alongside insulin therapy over a period of six months. Hypoglycemia is the most common short-term complication of T1D, and we need to do more to help people with T1D manage their blood sugar to reduce dangerous lows. This clinical trial is trying to do just that.
Based on observations from previous clinical studies, cadisegliatin may be able to lower the risk of hypoglycemia. Participants in the phase 2 SimpliciT1 study showed clinically meaningful improvements in blood sugar control in addition to a 40% reduction in hypoglycemic events compared to the placebo. Nearly 600 participants have received cadisegliatin so far—it’s been well-tolerated and hasn’t increased the risk of diabetic ketoacidosis (DKA).
Unexpected clinical trial results
Sometimes, clinical trials have unexpected results. In this case, researchers may pivot to change the design of future trials to ensure they’re looking for the right thing. For cadisegliatin, earlier trials primarily looked at improvements in control of high blood sugar, but researchers noticed significant reductions in hypoglycemic events, too. Now, this is the primary outcome for the CATT1 trial—while this wasn’t the original intention, it’s the most clinically meaningful assessment for people with T1D.
Dr. Klein, Kass, and their team are actively recruiting for the CATT1 trial over the next three or so months.
Some key eligibility criteria are:
- Minimum of five years since T1D diagnosis
- At least one moderate to severe hypoglycemic event (Level 2 or 3) in the last two months prior to screening
- 18 years of age or older
- HbA1c levels < 9.5%
- Use of a continuous glucose monitor (CGM) for at least three months prior to screening (excluding closed-loop systems)
Clinical classification of hypoglycemic events
| Level | Severity | Blood glucose range and characteristics |
|---|---|---|
| 1 | Mild | 54 to 70 mg/dL |
| 2 | Serious/moderate or clinically significant | < 54 mg/dL |
| 3 | Severe | An altered mental and/or physical state requiring immediate assistance to prevent progression to loss of consciousness, seizure, coma, or death. |
Participants, investigators, and care providers will all be unaware of whether the participants are receiving cadisegliatin or the placebo, which will both be given orally. Participants can expect a combination of in-person and virtual visits over a 26-week period. During these visits, the clinical research team will check blood sugar levels, perform other lab tests to assess safety, and provide top-tier T1D care.
At UNC, for the entire length of the trial, participants will have access to a dedicated study team including a nurse coordinator, research assistant, and diabetes specialists. The clinical research team will guide participants through every step of the process—answering questions, reviewing results, providing support—so that people with T1D and their families can feel confident that they are in good hands.
Interested in participating?
Complete a pre-screener questionnaire to see if you qualify!
The science behind cadisegliatin
We know that cadisegliatin has been shown to reduce the risk of hypoglycemia in people with T1D (SimpliciT1 study). The next question is: how?
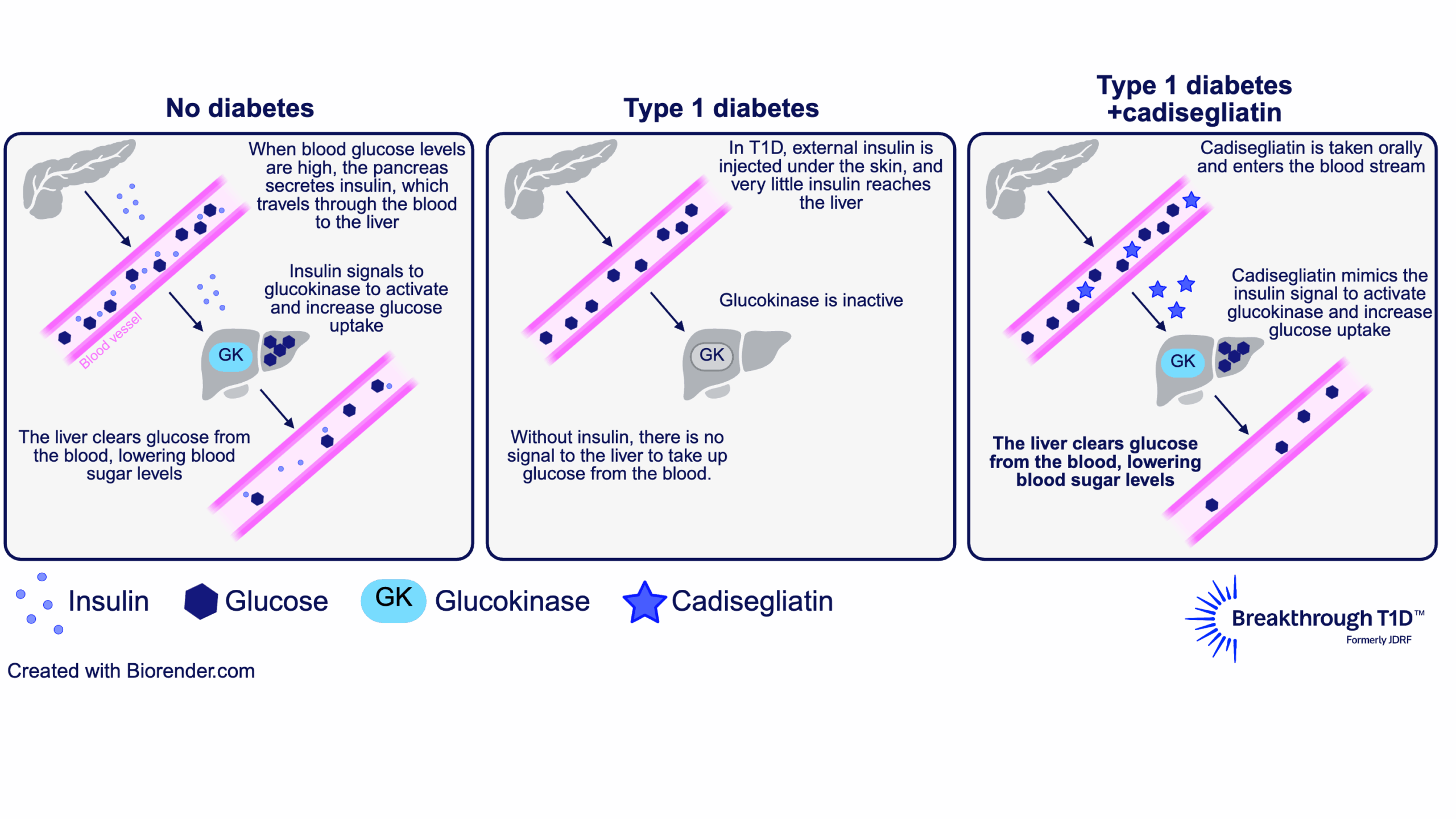
The answer to this question lies in the liver. The liver plays a major role in blood glucose control by taking up excess sugar from the blood and storing it until it’s needed again. To facilitate this, an enzyme called glucokinase in liver cells is activated in response to higher levels of insulin (indicating high blood sugar). This allows liver cells to remove glucose from the blood, bringing blood sugars down to a normal range.
Some of the glucose in the liver is also converted into glycogen, a storage form for glucose. This way, the liver can help increase blood sugar levels when needed, such as in between meals, overnight, or during exercise, to avoid low blood sugar. In this way, glucokinase helps reduce both hyperglycemia and hypoglycemia.
In people with T1D, the pancreas produces little to no insulin, and injected insulin doesn’t reach the liver the same way insulin from the pancreas does. So, glucokinase doesn’t get the signal that allows liver cells to respond to high blood sugar—making hyperglycemia and hypoglycemia worse.
This is where a glucokinase activator like cadisegliatin comes in. It keeps glucokinase in an active state, which restores the ability of liver cells to respond to (and reduce) high blood sugar. Then, in response to low blood sugar, the liver can release some of the sugar it has stored as glycogen into the blood stream. This is why cadisegliatin is being studied in people with T1D— it has the potential to improve blood glucose control in the liver and reduce the risk of highs and dangerous lows, with the goal of easing the daily burden of living with the disease.
Make a difference for yourself and others by participating in clinical trials
How to get involved
If you’re interested in getting involved at UNC, visit their Research for Me website to learn more about the CATT1 clinical trial at this location and other diabetes trials that may be the right fit for you. Reach out to the study team directly, and they will follow up with you to answer your questions and see if you qualify.
You can also register to be contacted by clinical researchers at UNC about participating in future studies for T1D. A research team member will reach out if a study becomes available that matches your profile criteria.
The CATT1 trial is also enrolling at other locations in the U.S. Find the trial site closest to you.
The clinical research team at UNC encourages trial participation as an opportunity to contribute to the body of research that may improve T1D care not only for yourself, but for the T1D community. Participants receive continued support at every step of the way—and safety is the highest priority.
By choosing to participate in a trial, people with T1D may gain access to research treatments, drugs, or devices that may make daily life with T1D easier, while receiving top quality diabetes care delivered by renowned medical centers. The data generated from these trials—thanks to the courageous and brave T1D community members who choose to participate—will shape the treatment landscape for future generations of people with T1D. Dr. Klein is one of many investigators who finds the dedication of the T1D community inspiring. “…I am consistently amazed and moved by the generosity of our participants who dedicate their time and energy to help move science forward,” she explains.

“The T1D community is incredibly generous with their time and motivated to help advance care—not just for themselves, but for the type 1 diabetes community and future generations of people who will live with type 1 diabetes. It’s really an honor to be able to work with them.”
-Klara Klein, M.D., Ph.D.
For Kass, it’s personal. By having T1D himself, he can form deeper connections with trial participants and their families. This allows him to keep conversations honest, participate in shared problem-solving, and understand the same day-to-day challenges of living with T1D—all a part of why he’s excited to be a member of the team providing top-tier diabetes care.
“Some of my favorite moments are when a participant or parent says, ‘You get it,’ and we can talk not just about the study, but about what it means to live well with diabetes. Whether it’s troubleshooting a CGM issue, celebrating a small win in blood sugar control, or simply being a dependable point of contact, I value the trust our participants place in us. That trust is what keeps me passionate about this work.”
-Alex Kass, MSN, MBA, RN, CDCES

Learn more about clinical trials
Visit Breakthrough T1D’s clinical trials web page to learn more about how you can get involved in clinical trials. Use our clinical trial matching tool to find recruiting trials near you that you may be eligible for. Connect with a Clinical Trial Education Volunteer in your area to better understand the process and get your questions answered.
Without clinical trials, progress wouldn’t be possible. All new therapies, devices, and treatments need to be tested to get from labs and into clinics as quickly and safely as possible. Thank you to every trial participant—in the past, present, and future—for helping us get closer to a world without T1D.
Analysis compiled by Avalere Health and supported by Breakthrough T1D (formerly JDRF) finds that research funded by the Special Diabetes Program (SDP) has yielded more than $50 billion in federal healthcare savings.
Created by Congress in 1997 and administered by the National Institutes of Health (NIH), the SDP provides federal funding to support research to prevent, treat, and ultimately cure type 1 diabetes (T1D).
The Special Diabetes Program (SDP) is set to expire soon! Ask your Members of Congress to renew the SDP!
Working closely with the SDP’s champions in Congress—Senators Susan Collins (R-ME) and Jeanne Shaheen (D-NH) and Diana DeGette (D-CO) and Gus Bilirakis (R-FL)—Breakthrough T1D leads advocacy efforts supporting continued funding for the SDP.
The SDP has directly supported many of the most important innovations that have changed how T1D is managed—including continuous glucose monitors (CGMs) and automated insulin delivery (AID) systems.
Today, these technologies are the standard of care and are used more broadly, including by people with type 2 diabetes (T2D) contributing to improved health outcomes for people with T1D and T2D.
Understanding the SDP’s Economic Impact
To better understand the program’s economic impact, Avalere Health conducted an analysis of estimated federal cost savings associated with SDP-supported technologies.
The analysis focused on direct medical expenditures and modeled the savings resulting from the use of CGM and AID systems including savings to the tax-payer supported public insurance programs like including Medicare, Medicaid, and the Department of Veteran’s Affairs.
Avalere Health’s findings indicate that CGMs and AID systems alone have generated at least $50 billion in federal healthcare savings through improved glucose management and reduced diabetes-related complications.
The actual federal savings and total economic impact of these technologies is likely higher as the analysis does not account for indirect cost savings—such as improved productivity (such as, not having to take time off work to treat a low, etc.), reduced disability, or long-term prevention of complications.
CGM and AID 2024 utilization and savings, total federal savings from start of SDP
| Technology | Population Using Technology (2024) | Annual Savings Per Patient (2024) | Federal Savings By Diabetes Type (1998-2024) | Total Federal Savings (1998-2024) |
|---|---|---|---|---|
| Continuous Glucose Monitors (CGMs) Without AID | T1D: 749,700 T2D: 1,738,600 | $5,502 | T1D: $10.9 Billion T2D: $21.2 Billion | $32.1 Billion |
| Automated Insulin Delivery System (AID) | T1D: 780,300 T2D: 1,856,400 | $5,306 | T1D: $5.4 Billion T2D: $12.9 Billion | $18.3 Billion |
T1D therapies on that are on the market or currently in development have benefited from SDP-supported research in numerous ways, including:
- Clinical trials and technology validation for CGMs and AID systems
- Development of disease-modifying therapies, including Tzield, the first therapy approved by the FDA to delay the onset of insulin therapy dependence in T1D
- Development of advanced therapies for diabetic eye disease
- Early-stage beta-cell replacement research, including Lantidra, the first therapy approved by the FDA to place insulin-producing cells back in the body and ultimately reduce or eliminate the need for insulin therapy
The SDP’s Future
This study and existing literature confirm that the SDP has demonstrated a strong return on investment—both clinically and economically. The SDP has also made life with T1D more manageable. AID systems are now standard of care and they are leading to better outcomes with less disease burden.
Ongoing support for diabetes research and treatments will be essential to realizing the full potential of emerging innovations for individuals with diabetes
Disruptions in SDP funding may hinder clinical research infrastructure, delay scientific advancement, and slow patient access to therapies that reduce complications and lower overall costs.
As policymakers evaluate the future of the SDP in the debate over government funding stakeholders should consider how funding decisions will maintain or interrupt momentum in diabetes research, bringing advanced therapies to market, and how that will impact continued cost savings from improved health outcomes for people with diabetes.
Read the Insights & Analysis report and the full white paper on the Avalere Health website.
Editor’s note: Content for this story drawn from Avalere Health’s Insights & Analysis report.
Continuous glucose monitors, or CGMs, have become increasingly popular with people who do not live with type 1 diabetes (T1D) as a way to observe the impact of activity and food on their blood-glucose levels.
While increased visibility of T1D technology can help normalize an advanced way to manage what is often an invisible condition, can wearing a CGM benefit people without T1D? Breakthrough T1D takes a look.
What are continuous glucose monitors?
Continuous glucose monitors are small, wearable devices that continuously measure a person’s blood-glucose levels. They are primarily used in place of glucose meters and finger pokes. A sensor just under the skin measures the glucose levels in real time. The levels are then relayed to a receiver, smartphone, watch, or insulin pump, which displays the readings.
How do CGMs benefit people living with T1D?
Continuous glucose monitors have been transformative for daily type 1 diabetes management. They have proven to:
- Lower HbA1c levels
- Reduce episodes of hypoglycemia (low blood sugar)
- Reduce the risk of complications associated with chronic hyperglycemia (high blood sugar)
- Increase time-in-range
Breakthrough T1D has played a pivotal role in the development and accessibility of CGMs for improved health outcomes in people living with type 1 diabetes.
In 2008, a Breakthrough T1D-funded clinical trial demonstrated the efficacy of CGMs in helping to manage blood sugar, with lower HbA1c levels and reduced rates of severe hypoglycemia in people living with T1D. This led to broader health insurance coverage and, ultimately, broader use of CGMs in the T1D community. The expansion of Medicare coverage of CGMs in 2022 also helped increase their use.
CGMs also have remarkable benefits during pregnancy with T1D. A 2017 study co-funded by Breakthrough T1D and the Canadian Institutes of Health Research showed that CGM use before and during pregnancy improves the health outcomes for both mothers and babies while reducing costs for neonatal hospitalization.
Today, eight CGMs are available in the U.S., and several are used in automated insulin delivery systems.
Unlocking access
Though T1D therapies like continuous glucose monitors are approved for use in the U.S., that isn’t enough. Breakthrough T1D tirelessly advocates for insurance coverage and the adoption of these therapies so that the entire T1D community can benefit from them.
Are there benefits to using a CGM if you don’t have type 1 diabetes?
For people without diabetes, CGM use is still considered investigational. “Early studies suggest CGMs may help detect abnormal blood sugar patterns in those with elevated risk—such as individuals who are overweight, have a strong family history of diabetes, or show borderline lab values—sometimes revealing glucose variability that standard tests might miss,” said Amin Ghavami Nejad, Senior Research Scientist at Breakthrough T1D.
There is also interest in using CGMs among athletes to monitor for exercise-related hypoglycemia and in exploring how blood sugar fluctuations may relate to cardiovascular risk. However, CGMs are not FDA-approved for individuals without diabetes, and there is no established clinical framework for interpreting the data in this population. More research is needed before CGM use in people without diabetes can be broadly recommended.
The role of continuous glucose monitoring in type 2 diabetes (T2D), however, continues to grow. The American Diabetes Association (ADA) 2025 Standards of Care recommended CGM use for adults with T2D who use insulin based on evidence that they improve glucose control, reduce hypoglycemia, and help patients make more informed daily decisions. The recent launch of Dexcom’s over-the-counter CGM, Stelo, also reflects rising interest in expanding access for people with T2D not using insulin and those with prediabetes, though this remains an evolving space and is not yet fully supported by clinical guidelines.
Breakthrough T1D will continue to support research in this area to accelerate therapies and access that can benefit those living with T1D and beyond.

ADA Recap Series
This article is the first of our three-part ADA Recap Series. Breakthrough T1D was on site in Chicago, IL from June 20-23 for the American Diabetes Association’s (ADA) 85th Scientific Sessions. We’re here to report on the latest-and-greatest type 1 diabetes (T1D) advancements—including many driven by Breakthrough T1D funding. Look out for tomorrow’s article for updates on Cures.
Improving Lives
Breakthrough T1D’s Improving Lives program focuses on devices, insulins, adjunctive therapies, treatments for complications, and psychosocial interventions to improve the health and quality of life of people living with T1D.
Adjunctive therapies and complications
There was significant focus on GLP-1 receptor agonists (GLP-1RAs) and SGLT inhibitors (SGLTi) in reducing long-term complications and improving glycemic control in people with T1D.

GLP-1 receptor agonists
Glucagon-like peptide 1 receptor agonists mimic the hormone GLP-1, which elevates insulin and regulates appetite. Examples include Ozempic® (semaglutide) and Mounjaro® (tirzepatide), which acts on both GLP-1 and a similar target, GIP.

SGLT inhibitors
Sodium-glucose cotransporter inhibitors target kidney cells to prevent them from reabsorbing glucose into the blood so it gets excreted as waste. Examples include Farxiga® and Zynquista®.
While SGLTi and GLP1-1RAs have proven effective for heart and kidney disease in type 2 diabetes (T2D) and in people without diabetes, people with T1D have often been excluded from critical trials. Thanks to years of advocacy and support from Breakthrough T1D, T1D trials are ongoing—and real-world evidence suggests that GLP-1RAs and SGLTi could be impactful in the T1D community as well.
Real-world evidence for GLP-1RA use in T1D
- Presenter: Ildiko Lingvay, M.D.; University of Texas Southwestern
- People with T1D have self-reported that they decided to try GLP-1RAs for weight loss and improved glycemic control.
- Real-world evidence suggests that GLP-1RAs have a clinically meaningful impact on weight and reduced insulin dose.
- While GLP-1RAs are generally safe, some people have stopped use because of gastrointestinal side effects. These side effects are also seen in people with T2D and people without diabetes.
A review of SGLTi and GLP-1RAs in reducing chronic kidney disease (CKD) in T1D
- Presenter: David Cherney, Ph.D.; University of Toronto
- In the EMPA-KIDNEY trial that included non-diabetes participants and people with T1D or T2D, empagliflozin (SGLTi) improved kidney health in people with T1D.
- In the ATTEMPT trial, dapagliflozin (SGLTi) improved time in range (TIR), reduced HbA1c levels, and had positive effects on kidneys in youth with T1D.
- The Breakthrough T1D-funded enrolling phase 3 SUGARNSALT trial is testing whether sotagliflozin (SGLTi) can prevent progression of moderate to severe kidney disease in people with T1D, and it includes careful diabetic ketoacidosis (DKA) risk mitigation strategies.
- The SEMA-AP trial found that semaglutide (GLP-1RA) increases TIR in people with T1D when used alongside an AID system.
- The Breakthrough T1D-funded recruiting phase 2 REMODEL-T1D trial is testing if semaglutide (GLP-1RA) can improve kidney health in people with T1D.

Glucokinase
Glucokinase (GK) is an enzyme in liver cells that works in an insulin-dependent manner to regulate blood sugar. In people with T1D who have little insulin reaching the liver, GK can’t work as normal, contributing to higher blood sugar.
Use of a glucokinase activator for glycemic control
- Presenter: Klara Klein, M.D., Ph.D.; University of North Carolina
- In the phase 1/2 SimpliciT1 study, people with T1D who received the GK activator TTP399 showed improvements in blood glucose with fewer hypoglycemic events.
- A different study found that TTP399 does not increase the risk for DKA.
- These studies were done in collaboration with vTv Therapeutics, a company with funding and support from Breakthrough T1D and the T1D Fund: A Breakthrough T1D Venture. The phase 3 CATT1 study for TTP399 is testing whether it can reduce moderate to severe hypoglycemic events in people with T1D.
Adjunctive therapies and complications highlight: Breakthrough T1D-funded research
Halis Kaan Akturk, M.D. (University of Colorado), Janet Snell-Bergeon, Ph.D., MPH (University of Colorado), and Viral Shah, M.D. (Indiana University) presented findings from the Breakthrough T1D-funded ADJUST-T1D clinical trial, which was recently published in the New England Journal of Medicine Evidence. The trial tested whether semaglutide (GLP-1RA) can improve glycemic and weight outcomes in adults with T1D and obesity who are using an AID system. 36% of people treated with semaglutide met the primary endpoints of TIR greater than 70%, time below range less than 4%, and weight loss of 5% or more compared to the placebo, and the drug was well-tolerated and safe. This trial represents critical evidence for use of a GLP-1RA as a potential way to manage both glycemic control and weight in people with T1D.
Ye Je Choi, MPH (University of Washington) reported on the CROCODILE study, which examined metabolic alterations in kidneys of people with T1D. She observed early structural and metabolic changes in kidneys that occurred before the onset of clinical kidney disease and associated structural damage. Her work could contribute to the development of biomarkers that can help predict the onset of kidney disease in people with T1D before it occurs.
Jeremy Pettus, M.D. (University of California at San Diego) conducted a phase 2 clinical trial to address insulin resistance in people with T1D. External insulin therapy can increase levels of insulin in the blood relative to glucose, which reduces sensitivity and may contribute to cardiovascular disease (CVD). Treatment with the glucagon receptor antagonist volagidemab, which prevents the liver from releasing glucose into the blood, reduces insulin requirements by 15%, resulting in improved glycemic control and insulin sensitivity without changes in bodyweight.
Schafer Boeder, M.D. (University of California at San Diego) worked with Dr. Pettus on a phase 1/2 clinical trial that tested whether the addition of SGLTi to the glucagon receptor antagonist volagidemab can further improve glycemic control in people with T1D. The combination of therapies increased TIR up to 86% from 70% and reduced daily insulin use by 27%. Further research is needed to better understand the safety profile of this regimen.
Justin Gregory, M.D. (Vanderbilt University) worked with Dr. Pettus and Dr. Boeder on the above study. He also presented on the use of GLP-1RAs and dual GLP-1/GIP receptor agonists for reducing complications in T1D.
Key takeaways
Clinical trials with GLP-1RAs and SGLTi are providing encouraging evidence about these therapies’ potential to improve long-term health in people with T1D. Breakthrough T1D is working toward a future where these drugs are an option for people with T1D to better manage their blood sugar and reduce complications.
Devices
Real-world insights from Automated Insulin Delivery (AID) systems
- Presenter: David Maahs, M.D., Ph.D.; Stanford University
- Based on published real-world evidence for AID systems in people with T1D, TIR is increased by an average of eight to 15% from baseline in a range of studies across various systems.
- Youth with T1D have better glycemic control and reduced rates of DKA with AID systems. Those with lower TIR at the start of AID system use see the greatest improvements.
Real-world evidence: iLet Bionic Pancreas AID system
- Presenter: Steven Russell, M.D., Ph.D.; Beta Bionics
- The iLet Bionic Pancreas contains a continuously adapting algorithm that automatically determines insulin doses. No carbohydrate counting is required, and meals are only announced as breakfast, lunch, and dinner.
- Data was collected from 16,000 users over two years.
- Users achieved an average HbA1c level of 7.3%, down from 8.9%. This is accompanied by low rates of hypoglycemia and significantly improved self-reported quality of life.
Continuous ketone monitoring: Innovations and clinical applications
- Presenters: Ketan Dhatariya, MBBS, M.D., Ph.D. (Norfolk and Norwich University Hospitals), Lori Laffel, M.D., MPH (Harvard University), Jennifer Sherr, M.D., Ph.D. (Yale University), and Richard Bergenstal, M.D. (HealthPartners Institute).
- DKA rates are increasing in the U.S., but mortality rates from DKA are decreasing.
- The history of continuous glucose monitoring (CGM) offers a roadmap for continuous ketone monitoring (CKM) development, showing how early skepticism gave way to broad clinical impact.
- CKM could allow for earlier detection of rising ketones to prevent DKA. CKM also has the potential to identity infusion set failures, be a valuable addition to AID systems, help monitor early-stage T1D, and more.
- Five new studies funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) will develop CKM for safe and effective use of SGLTi in T1D.
- Tandem, Beta Bionics, Sequel MedTech, and Ypsomed announced plans to integrate Abbott’s dual glucose ketone sensor into their AID systems.
Making the case for time in tight range
- Presenter: Gregory Forlenza, M.D.; University of Colorado
- Dr. Forlenza presented on the benefits and challenges of time in tight range (TITR), also known as time in normal glycemia (TING), defined as blood glucose levels between 70-140 mg/dL.
- TITR will likely be more clinically beneficial than TIR as fluctuations outside of TITR may be better predictors of complications and offer a better therapeutic window for intervention.
- More research is needed to advance therapeutics that will allow people with T1D to achieve TITR before it can be integrated into clinical decisions.
Devices highlight: Breakthrough T1D-funded research
Erin Cobry, M.D. (University of Colorado) presented the results of a Breakthrough T1D-funded clinical trial evaluating an artificial intelligence-powered AID algorithm designed to not require meal announcements. She showed that this algorithm (used without meal announcements) improved overnight TIR, and provided equivalent daytime TIR, compared to participants’ usual care. A major goal for Breakthrough T1D is to advance AID systems that do not require meal announcements to improve both glucose outcomes and quality of life for people with T1D.
Key takeaways
Devices have transformed how this disease is managed. Systems are becoming easier to use with less user input—and, critically, people with T1D are doing better. This is the dream Breakthrough T1D had when we launched the Artificial Pancreas project 20 years ago. We will continue to drive toward our goal of developing systems that provide superior health outcomes with minimal user burden.
Insulins
Inhaled insulin treatment for youth with T1D
- Presenter: Michael Haller, M.D.; University of Florida
- Afrezza® is an inhaled, fast-acting insulin that has proven to be effective in adults.
- The phase 3 INHALE-1 study examined Afrezza® in youth with T1D. Users report greater treatment satisfaction and no increase in weight compared to injected rapid-acting insulin analogs.
- Afrezza® is safe for youth with T1D. The most common adverse events were pulmonary-related, such as coughing.

Breakthrough T1D’s Improving Lives team making an impact
Courtney Ackeifi, Ph.D., Senior Scientist, hosted an Improving Lives Happy Hour with Breakthrough T1D-funded researcher Jeremy Pettus, M.D. The discussion included research priorities for adjunctive (non-insulin) therapies for people with T1D and their healthcare providers. They also discussed the importance of industry partnerships and the role of Breakthrough T1D in driving these relationships, which can accelerate new T1D therapies toward the clinic.
Dr. Ackeifi also spoke at the ADJUST-T1D trial update, contextualizing the use of adjunctive therapies like GLP-1RAs for superior glucometabolic control in people with T1D.
Look out for tomorrow’s article for an update on Cures research presented at ADA 2025!
Menstrual cycles and type 1 diabetes (T1D) have a lot in common: they can be unpredictable, inconsistent from person to person, and physically challenging. For Women’s Health Month, Breakthrough T1D is taking a closer look at how periods and menopause may influence blood sugar.
What happens during the menstrual cycle?
Most girls get their first period around age 12. Then, each month, the ovary releases an egg; if it doesn’t get fertilized, it’s reabsorbed, and the lining of the uterus is shed as a period. This cycle continues unless the egg is fertilized, after which it implants into the uterine lining (and periods stop). At the end of a woman’s reproductive window between ages 45 and 55, known as menopause, periods stop permanently, and pregnancy is no longer possible.
These complex processes are driven by a conversation between a woman’s brain, ovaries, and uterus, which is controlled by different hormones released at different times. These hormones—such as progesterone and estrogen—may significantly impact blood sugar.
Life with T1D and periods
Women with T1D are more likely to experience longer and irregular periods. More than one-third of teenage girls with T1D—especially those diagnosed before their first period—may have abnormal menstrual cycles. Because 50-60% of people are diagnosed before the age of 15, many women and girls will experience this.
The hormonal culprits responsible for these irregularities affect blood sugar differently depending on the menstrual cycle stage. Progesterone, which is elevated leading to the next period, may increase insulin resistance and the risk of hyperglycemia, especially compared to after the period ends.
The role of estrogen is less clear: it may increase insulin sensitivity—especially when levels are rising post-period—or have no effect at all.
Pregnancy Guide
Get guidance and support on all stages of pregnancy, from pre-pregnancy
planning to juggling T1D as a new mom.
What about menopause?
On average, women diagnosed with T1D before their first period may have a three-year reduction in reproductive lifespan compared to women without T1D—meaning earlier menopause.
As if the barrage of symptoms—including mood swings, brain fog, and sleep issues—weren’t enough, women with T1D might encounter unpredictable blood sugar because of decreasing estrogen and progesterone. Even more, menopause and hypoglycemia can have overlapping symptoms, so special attention is needed to prevent dangerous lows.
Putting it all together
While further scientific studies are required to better understand how hormonal changes affect T1D, there are things you can do to manage your blood sugar more easily.
First, work together with your healthcare team to track blood sugar levels during different menstrual cycle phases or menopause to identify patterns and know what to expect. Women with T1D who take hormonal birth control may have different considerations.
Next, the usual: exercise, eat healthy, and get good sleep. Carefully consider your diet right before your period; while you may crave sugar and carbs, you may also be more susceptible to hyperglycemia, so proceed with caution.
Finally, be kind to yourself. Life with T1D can be hard. Periods and menopause can be hard. Just remember—you’re not alone!
They’re all over the news: Ozempic ®. Trulicity ®. Jardiance ®. Mounjaro ™. And more.
These drugs are all approved for glucose control in type 2 diabetes (T2D). Some of them also have additional indications reflecting their demonstrated benefits for cardiovascular disease, kidney health, and obesity.
None of these drugs are currently approved for people with type 1 diabetes (T1D). However, a recent consensus report addressed the growing interest in GLP-1 receptor agonists as an adjunctive therapy for T1D and their “potential to improve glycemic and metabolic outcomes without increasing the risk of severe hypoglycemia or diabetic ketoacidosis.”
Let’s examine GLP-1 therapies and SGLT inhibitors and consider how drugs like Ozempic may one day help people with type 1 diabetes.
GLP-1 therapies
GLP-1 (glucagon-like peptide-1) receptor agonists work in multiple ways to control blood glucose and obesity. They block the release of glucagon, stimulate insulin production, slow the rate at which your stomach empties, and increase the sensation of feeling full. They are usually injected, but oral versions are available.
In people with T2D, this class of drugs lowers blood sugar levels and, for most people, causes weight loss. GLP-1 therapies have also been shown to reduce the risk of long-term cardiovascular complications often experienced by people with T2D, such as heart attack and stroke.
GLP-1 drugs include Ozempic/Rybelsus/Wegovy (semaglutide), Trulicity (dulaglutide), Victoza (liraglutide), and Mounjaro (tirzepatide), among others.
When GLP-1 treatments hit the market in the early 2000s, Breakthrough T1D and others funded several clinical trials to test whether GLP-1 receptor agonists, in addition to insulin, improved outcomes for people with type 1 diabetes. While some of these studies showed that the addition of GLP-1 therapies to insulin led to improvements in HbA1c, total insulin dose, and weight, the benefits were relatively modest and accompanied by some side effects like hypoglycemia. As a result, these studies did not lead to GLP-1 receptor agonists being highly adopted for use by people with T1D.
However, these trials were done with older GLP-1 drugs. We are investigating whether the newest, most advanced GLP-1 therapies (like Ozempic) will improve the health of people living with type 1 diabetes. (See below!)
SGLT inhibitors
SGLT (sodium-glucose co-transporter) inhibitors are oral medications for people with T2D that lower blood sugar by preventing the kidneys from reabsorbing glucose, leading to the excretion of sugar through the urine.
In addition to improving blood sugar for people with and without T2D, these drugs also provide benefits such as weight loss, blood pressure reduction, and transformative benefits to the heart and kidneys.
SGLT drugs include Jardiance (empagliflozin), Farxiga (dapagliflozin), and Invokana (canagliflozin), among others. Despite demonstrating improved glucose control for people with type 1 diabetes, SGLTs have not been approved for people with T1D in the U.S. Increased risk of diabetic ketoacidosis (DKA) in this population limits the use of these therapies. A key Breakthrough T1D priority is to find ways to mitigate this risk so people with T1D can also take advantage of the SGLT cardiovascular and renal benefits.
Breakthrough T1D-funded research in GLP-1 and SGLT therapies
Breakthrough T1D has a long history with GLP-1 medications like Ozempic. In the 1980s, Breakthrough T1D-funded researcher Pauline Kay Lund, Ph.D., was the first to clone the hormone glucagon and discover two new hormones, one of which was GLP-1.
Today, there is real-world evidence of GLP-1 receptor agonists improving the lives of people with type 1 diabetes.
Real-world evidence
Observational studies using historical patient data from electronic health records (not randomized clinical trials) that can give an idea if a drug might be beneficial or not for a certain indication.
Evidence demonstrates that semaglutide (Ozempic) or tirzepatide (Mounjaro) have the potential to lower A1c, increase time-in-range, and reduce the amount of daily insulin needed in people with T1D. More research is needed in this area. That’s where Breakthrough T1D comes in!
Breakthrough T1D-funded research on GLP-1 and SGLT therapies is investigating the benefits of these drugs for people with T1D, including reducing the risk of common complications of type 1 diabetes like cardiovascular disease and kidney disease.
Snapshot of active clinical trials in GLP-1 and SGLT therapies
Here are a few examples of Breakthrough T1D-funded clinical trials in GLP-1 and SGLT therapies that are currently underway:
| Clinical Trial Name | Study Details |
|---|---|
| REMODEL T1D | Determine whether semaglutide (Ozempic) protects the kidneys in those living with T1D. |
| Triple Therapy in T1DM | Assess whether the addition of dapagliflozin (Farxiga) to semaglutide (Ozempic) and insulin improves glycemic control in those living with T1D. |
| SUGARNSALT | Determine the effectiveness and safety of sotagliflozin (Inpefa) in slowing kidney function decline in those living with T1D and moderate to severe diabetic kidney disease. |
| Dapagliflozin + Pioglitazone in T1D | Examine how adding dapagliflozin (Farxiga) and pioglitazone (Actos) to insulin therapy affects glucose control and ketone concentration in people living with T1D. |
Our commitment to improving lives
Breakthrough T1D strives to improve health outcomes in people living with type 1 diabetes. Insulin therapy alone is often not enough for people with T1D to achieve glucose and metabolic control. The use of adjunctive drugs that complement insulin therapy can help. Since the FDA has already approved these medications for treating other conditions, positive results from these clinical trials could speed up the adaptation of these therapies for people living with T1D.
Learn more about clinical trials and how they are advancing breakthroughs for the T1D community.
While we look back on 2024, we can reflect upon the incredible progress we’ve made in advancing breakthroughs toward cures and improving everyday life with T1D.
This wouldn’t have been possible without each and every one of you and your continued support of our mission as we drive toward cures for T1D.
Here are the top 11 advances that together, we made happen in 2024:
Breakthrough T1D announced the launch of Project ACT, an initiative aimed at accelerating breakthroughs in T1D cell replacement therapies that do not require broad immunosuppression. Recent advances, such as Vertex’s stem cell-derived islets, have been made possible by Breakthrough T1D’s Cell Therapies program as part of our drive toward cures. The goal of Project ACT is to push research, development, regulatory policies, access, and adoption to increase the rate at which cell therapies without the need for broad immunosuppressants will become available to people with T1D.
Why this matters: Immunosuppressive drugs are a barrier to access to cell replacement therapies because of their toxic side effects, which is why islet transplants are currently only available to people with severe low blood sugar (hypoglycemic) unawareness and episodes. By striving toward a future where we realize the benefits of cell replacement therapies without the downsides of the current regimen of immunosuppressants, we will make islet replacement therapies broadly accessible to the T1D community.
Vertex’s clinical trial of VX-880, a first-generation stem cell-derived islet replacement therapy for people with severe hypoglycemia (requiring the use of immunosuppressants), has transitioned into a phase 1/2/3, or pivotal, trial. This news comes after Vertex shared incredibly promising data in the earlier phases of the trial, including 11 of 12 participants reducing or eliminating the need for external insulin.
The upcoming trial will expand to 50 people who will get a single, target dose of VX-880. The primary endpoint will be insulin therapy independence without severe hypoglycemic events after one year. This is the final clinical testing stage before Vertex can seek FDA approval.
Breakthrough T1D has a decades-long relationship with Vertex and the leading scientists behind stem cell-derived islet therapies, an advancement that would not have been possible without Breakthrough T1D funding and support. The T1D Fund had invested in Semma Therapeutics, which was acquired by Vertex Pharmaceuticals in 2019, eventually leading to the active clinical development of VX-880 in T1D.
Why this matters: This is the first time a scalable cure for T1D is entering phase 3 clinical trials—a significant win and a huge step toward accelerating the delivery of cell therapies to members of the T1D community!
Tegoprubart: Transplant Survival Without Standard Immunosuppressive Drugs
Tegoprubart, an anti-CD40L immunotherapy that limits the immune response, is being tested in a Breakthrough T1D-funded study in people with T1D and severe hypoglycemia who have received deceased donor islets. Eledon Pharmaceuticals announced promising initial results in which two of three people achieved insulin therapy independence. According to the study, tegoprubart is safer for both people and transplanted cells in comparison to broad immunosuppressants, with milder side effects and greater islet survival. To further support this effort, the T1D Fund: A Breakthrough T1D Venture invested in Eledon.
Cell Pouch: A Home for Transplanted Islets
Breakthrough T1D has been supporting the development of Cell Pouch, an implantable device from Sernova that provides a safe, immune-protected environment for transplanted islet cells. In phase 1/2 clinical trials, all six people who received donor islets within the Cell Pouch achieved sustained insulin therapy independence with immunosuppressants, including long-term islet survival and function over five years without harmful side effects.
Why this matters: Standard of care immunosuppressive drugs that help avoid transplant rejection come with unwelcome side effects, such as increased risk of infection and malignancy and toxicity to kidneys, nerves, and islet cells themselves. Breakthrough T1D is focused on finding alternative ways to keep transplanted islet cells alive and healthy so that cell replacement therapies can become more tolerable and accessible.
In a major effort spearheaded by Breakthrough T1D, the first internationally recognized clinical guidelines for those who test positive for T1D autoantibodies have been published. These include guidance on monitoring frequency, education, and psychosocial support in addition to recommended actions for healthcare professionals (HCPs) when the risk of T1D progression is high. The guidelines were cooperatively developed with over 60 international experts spanning ten countries.
Why this matters: Previously, there had been no consensus on monitoring guidelines for people who test positive for T1D autoantibodies. Standardization of clinical recommendations means that individuals, families, and HCPs have tangible next steps to monitor early T1D progression and catch life-threatening complications sooner.
- Breakthrough T1D is leading a campaign to secure a recommendation for T1D screening from the U.S. Preventative Services Task Force (USPSTF), the main authority for preventative care. Approval would require T1D screening to be covered by insurance—an important step forward in expanding access.
- Driven by Breakthrough T1D’s advocacy efforts, The Centers for Medicare and Medicaid Services (CMS) established a unique ICD-10 code for stage 2 T1D. ICD-10 codes are used by HCPs to classify and document diagnoses, symptoms, and procedures. These codes provide a unified way for doctors and providers to indicate what diseases or conditions a person has in their electronic health record (EHR), empowering HCPs to document accurate diagnoses and provide the best possible care.
Why this matters: T1D early detection is critically important to prevent life-threatening complications at diagnosis and to give people necessary resources to make informed decisions about their health. Integrating T1D screening into the U.S. healthcare system will increase access to care.
The past year has seen some important advances in glucose management therapies and devices:
- Cadisegliatin, an activator of a blood sugar regulator in the liver, is being investigated in a phase 3 clinical trial (TTP399) as an adjunct therapy to insulin for people with T1D, although it is currently placed on clinical hold. vTv Therapeutics, the trial sponsor, is also a T1D portfolio company.
- The Omnipod 5 app is now available for the iPhone, making it easier to control the Omnipod without the need to carry a controller. It can also integrate with the Dexcom G6 continuous glucose monitor (CGM).
- Eversense 365 is the first FDA-approved year-round sensor that can easily integrate with automated insulin delivery (AID) systems. Other sensors require replacement after 10-14 days.
Why this matters: While advancements in glucose management have been pivotal in improving health outcomes for people with T1D, access remains a challenge. AID systems are globally underutilized, and not everyone has the necessary technology to connect devices. Breakthrough T1D is working to not only support advances in glucose management but also increase access.
Related content: While Breakthrough T1D consistently strives to improve the lives of those living with T1D, as an organization we have made incredible progress in the development of AID systems, also called the artificial pancreas systems. Read a historical perspective written by Breakthrough T1D volunteer Doug Lowenstein that covers conception to FDA approval of the first artificial pancreas systems, which changed the lives of people with T1D.
An inquiry spearheaded by the Breakthrough T1D affiliates in the U.K. uncovered risks of developing T1D eating disorders (T1DE), including bulimia, anorexia, or insulin restriction to lose weight. There is a significant gap in education and clinical guidelines for HCPs, a lack of internationally recognized criteria for T1DE diagnosis, and insufficient care integration, leading to preventable complications and healthy years of life lost. Breakthrough T1D recognizes the importance of spreading awareness and support for T1DE, and much work is needed to improve the lives of those living with T1DE.
Why this matters: There is an urgent need to change the way T1DE is approached, including integrated physical care with mental health services to get people with T1DE the access to care that they need.
In a study that included people with T1D, finerenone (Kerendia®) has been shown to improve cardiovascular outcomes in adults with heart failure. The drug is already approved in the U.S. to treat kidney and cardiovascular disease in people with T2D. Based on these results, Breakthrough T1D is supporting a clinical trial (FINE-ONE) in conjunction with Bayer to investigate the use of finerenone for T1D with the hopes of reducing kidney complications.
Why this matters: Kidney and cardiovascular disease remain significant challenges for those with T1D, especially given the FDA’s recent rejection of an SGLT inhibitor to lower blood glucose in people with T1D and chronic kidney disease. Yet, a new clinical trial (SUGARNSALT) will better assess the benefits versus risks.
Breakthrough T1D is advocating for the regulatory approval of C-peptide, a biomarker for insulin production by beta cells, to be used as an endpoint in clinical trials. An endpoint can accurately predict a meaningful benefit in clinical trials for disease-modifying therapies (DMTs; treatments that can slow, halt, or reverse T1D). To support this endeavor, Breakthrough T1D scientists and an expert consensus panel published research with evidence supporting C-peptide as an endpoint. Breakthrough T1D is continuing to engage with regulators, coordinate with industry, and assess more clinical trial data to drive this effort forward.
Why this matters: Current clinical trial endpoints (HbA1c, hypoglycemia, and complications) are not the best way to gauge the clinical benefits of T1D therapies. If C-peptide gets regulatory approval to be used as an endpoint, clinical trials could be smaller and shorter while still accurately assessing the advantages of a DMT. This means that drug development can move more quickly, and people with T1D will be able to access therapies sooner.
Related content: Two years ago, the T1D community received the incredible news that Tzield® had become the first FDA-approved disease-modifying therapy that can significantly delay T1D onset. Breakthrough T1D volunteer Doug Lowenstein recounts the life-changing drug’s journey nearly 100 years after the discovery of insulin.
The T1D Index is a data simulation tool that measures the global health impact of T1D, bridging gaps in our knowledge of public health statistics. T1D Index 2.0 has new and improved functionality, including advanced simulation capabilities, validation of data, and enhanced user experience. Breakthrough T1D contributed to both the development and improvement of the T1D Index.
Why this matters: The T1D index is critical in defining the intercontinental scope of T1D, driving us toward country-specific solutions and improved global health outcomes.
Earlier this year, JDRF rebranded to Breakthrough T1D. While our mission remains the same, our name needs to better reflect who we are and where we’re going. Our new brand aligns with our mission to accelerate life-changing breakthroughs for those of every age living with T1D as we work toward a world without it.
Why this matters: The proof is in the name—each day we strive to increase and accelerate breakthroughs in T1D, and it’s critical for our brand to accurately reflect our mission.
It’s certainly been an exciting year! While we still have more work to do, it’s crucial to celebrate our wins, both big and small, to see how far we’ve come in our push to make T1D a thing of the past.
Together, we’re accelerating breakthroughs for people with T1D, and the support of the T1D community drives our mission forward every single day, leading the way to lifechanging therapies and cures. Let’s see what 2025 has in store!
Update – November 22, 2024
This morning, Lexicon Pharmaceuticals, Inc. (Lexicon) announced they received a “deficiencies preclude discussion” letter from the FDA regarding this drug application. The FDA is not approving sotagliflozin for use in glucose control in people with T1D and CKD and there will be no further discussion in regards to this application.
As a result, Lexicon announced that they will stop pursuing sotagliflozin for use in glucose control in people with T1D and CKD.
Breakthrough T1D is extremely disappointed with this decision. Our funded research exploring the use of sotagliflozin in people with T1D will continue, and we will continue to push on multiple fronts for therapies, like this one, that can help address the burden and unmet needs of the T1D community.
There is a deep unmet need for therapies in addition to insulin for glucose control in people with T1D. This is especially true for those with additional complications like CKD. Today, a U.S. Food and Drug Administration (FDA) advisory committee recommended against the agency approving Lexicon’s sotagliflozin for blood glucose control in individuals with T1D and CKD. Breakthrough T1D believes this drug has benefits for people with T1D and those benefits outweigh the risks.
The case for more therapies
For over 100 years, insulin therapy has been the main treatment for T1D. Better insulin options and new technologies, like continuous glucose monitors and automated insulin delivery systems, have improved health and quality of life for many people with T1D. There is nothing that people with T1D cannot accomplish. But, by nearly every health-related quality of life metric, people with T1D score lower than individuals without T1D. This includes general health, mental health, vitality, physical functioning, social functioning, and others.
If you look specifically at T1D metrics, a similar picture appears. Only 26% of individuals with T1D achieve an HbA1c level under 7%, the American Diabetes Association’s recommended HbA1c target to reduce the risk of long-term diabetes-related complications like cardiovascular and renal disease and diabetic retinopathy.
Despite the significant advances that Breakthrough T1D has been instrumental in realizing, people with T1D are not doing well enough. We need tools that make it easier to do better.
What is sotagliflozin?
Sotagliflozin is an SGLT (sodium-glucose co-transporter) inhibitor. SGLT inhibitors are oral medications originally developed for people with type 2 diabetes (T2D). They lower blood sugar by preventing the kidneys from reabsorbing glucose, leading to the excretion of sugar through the urine.
In addition to improving blood sugar, this class of drugs provides transformative benefits and have been approved for heart and kidney health in people with T2D and people without diabetes. In addition, they promote weight loss and blood pressure reduction.
Benefits of sotagliflozin for people with Chronic Kidney Disease
It has long been recognized that maintaining tight glucose control is crucial for preventing chronic kidney disease (CKD) in individuals with T1D. Recent data indicate that for those with T1D who already have CKD, there is a significant correlation between high HbA1c levels and an accelerated loss of kidney function. Therefore, effective glucose management is vital for individuals with both T1D and CKD to avert serious health complications.
In addition to improving glucose control, sotagliflozin has shown improvement in CKD outcomes in large trials of people with T2D. It is reasonable to expect these benefits would also be seen in those with T1D who also have CKD. While we do not yet have data on long-term kidney outcomes associated with sotagliflozin in T1D, we are encouraged by data demonstrating that sotagliflozin improves key biomarkers of kidney function in people with T1D.
The risk of diabetic ketoacidosis
There is one key consideration for the safe use of sotagliflozin: the risk of diabetic ketoacidosis (DKA).
Using SGLT inhibitors can increase the risk of DKA in people with T1D, so it’s important to monitor and manage this risk. In 2018, experts came together to address these concerns. Their findings led to an international agreement in 2019 on how to reduce the risk of DKA in people with T1D who are using SGLT inhibitors.
However, DKA is a risk for everyone with T1D, not just those taking SGLT inhibitors. It’s important to be aware of DKA as a regular part of managing T1D.
Breakthrough T1D has long supported research to advance safe, effective use of SGLT inhibitors in T1D
Breakthrough T1D’s Improving Lives program supports the development of SGLT inhibitors for T1D; this includes trials to assess benefits and risks of this drug class in T1D, as well as device-based and other strategies for DKA risk mitigation. SGLT inhibitors are FDA approved for T2D and certain non-diabetic populations such as those with CKD or heart failure, and they are used by some people with T1D off-label. Our work supports studies to build a body of evidence that will allow people with T1D to improve health outcomes through safe and effective use of SGLT inhibitors.
What comes next?
Breakthrough T1D believes the benefits outweigh the risks for people with CKD and T1D and sotagliflozin should be approved. That’s why Sanjoy Dutta, Ph.D., Breakthrough T1D Chief Scientific Officer, delivered public comments in support of the drug’s approval today.
As the formal review process moves forward, Breakthrough T1D will continue to invest in research to develop therapies for kidney disease in T1D that can help people live longer and healthier lives. While the advisory committee’s recommendation plays a critical role, it is not the only consideration in the final decision of approval (or not). The overwhelming majority of people with diabetes and world-renowned healthcare providers who commented were in agreement with Breakthrough T1D that the drug should receive approval.
The FDA are required to make a decision by December 20, 2024.
Editor’s note: Written by guest blogger Matthew Tilton, D.O. In addition to being a physician, he is the father of 9-year-old Adalyn (pictured above), who lives with type 1 diabetes (T1D). Read his previous story for Breakthrough T1D.
As summer draws to a close and the back-to-school season begins, many parents are busy preparing their children for a new academic year. For many families, this requires a few more checkboxes on the list. My daughter Adalyn, who has type 1 diabetes (T1D), requires more than the usual school supplies and new clothes. Our back-to-school checklist includes items like insulin, glucose monitors, and an emergency care plan, all essential for her well-being.
As a parent of a child with T1D, the transition to a new school year brings a mix of emotions. There’s the usual excitement and straight-up anxiety. Will the teachers have questions? What will her classmates say? These are the questions that linger in my mind as the school year approaches. It turns out I am more anxious than she is.
The importance of a well-coordinated support system
Adalyn, however, is determined to approach the new school year with confidence. “I am a little nervous, but not much. I know my nurse is there to help me,” she said recently. Her words are a reminder of the importance of having a well-coordinated support system in place. Making sure the school nurse knows Adalyn’s care plan and is prepared to respond to any situation is a critical step in ensuring her safety.
Related content: School nurses have incredible impact
Additionally, we’ve worked closely with her teachers to make sure they’re aware of her condition and know how to take the right steps now to ensure her success later. This includes understanding the signs of high or low blood sugar and knowing when to allow her a break or snack. If you are looking for permission to be “that parent,” here you go. Be that parent. As a physician, occasional coach, and father, I have never once thought, “Oh, I really regret being too prepared for this.” I am sure I may be a headache, but it is this collaboration between parents, healthcare professionals, and educators that forms the foundation of a safe and supportive learning environment.
Creating an environment for thriving
Let’s not forget that continuous glucose monitors and insulin pumps have been life-changing. I’m thankful for the incredible advancements in medical technology that allow Adalyn to lead a relatively normal life. These tools enable her to do things she loves, like playing softball and other sports. But these tools are only part of the equation. The real challenge lies in creating an environment where she can thrive academically and socially, without constant worry.
This is where awareness and education become crucial. Schools must be equipped with the knowledge to support students with T1D, with the necessary medical supplies, and foster an environment where every child feels safe and understood. I have it on good authority teachers aren’t trained on T1D and all that comes along with it. That is where we—the prepared parents—come in. Teachers, staff, and even fellow students need to understand what T1D is and how they can help. A simple understanding can go a long way in making sure children like Adalyn feel safe and supported.
Setting our schools—and kids—up for success
If there’s one thing I’ve learned, it’s that managing type 1 diabetes is a team effort. It requires not just the dedication of parents and medical professionals, but also the understanding and cooperation of educators and community members.
So, as we prepare for another school year, I want to make a call to action. Let’s set our schools—and our kids—up for success. Let’s ensure that our schools are prepared to support children with T1D and other chronic conditions. This means advocating for better training for school staff, ensuring that schools have the necessary medical supplies, and fostering an environment where every child feels safe and understood.
As we send our children back to school, let’s also commit to sending them into environments where their health needs are met with compassion and competence. Let’s work together to ensure that every child, regardless of their medical condition, has the opportunity to learn and grow in a safe and supportive setting. This is not just about managing a disease; it’s about empowering our children to live their fullest lives. And until there are cures, we parents and caregivers must be the advocates, the educators, and the support system they need.
Together, we can make a difference
Breakthrough T1D offers a school guide to help parents advocate for their children’s needs. They also have resources to help educators understand the unique needs of students with T1D. Consult their T1D Resource Library for the school guide and other helpful life with T1D guides.
The road ahead is challenging, but we are not alone. Together, we can make a difference. Just like Adalyn confidently steps up to every challenge, we, too, must step up, ensuring that every child with T1D has the chance to thrive, both in school and in life.
The American Diabetes Association’s 84th Scientific Sessions is here! Scientists will present the latest type 1 diabetes (T1D) research, from early detection to glucose control to complications, all with the goal of improving lives for the T1D community.
- In November 2022, the FDA approved Tzield™ (teplizumab-mzwv) for use in delaying the onset of clinical T1D. With the availability of a treatment option for people with Stage 2 T1D, the field has changed its outlook on delay and prevention and navigating pediatric T1D, especially in the early stages. Annette-Gabriele Ziegler, M.D., presented on several screening programs in Europe, including Fr1da, which has screened 200,000+ pediatric participants and found that it significantly reduces DKA onset at clinical diagnosis, and GPPAD, which identifies infants with an elevated genetic risk of developing T1D and enrolls them in primary prevention clinical trials. Andrea Steck, M.D., highlighted the value of CGM-based metrics in evaluating T1D risk and R. Brett McQueen, Ph.D., discussed the economics of early detection.
At-risk, or Stage 2 T1D, means that a person exhibited 2+ T1D-related autoantibodies—antibodies against one’s own self—and their blood glucose is starting to be abnormal, but they are not yet insulin dependent. When someone becomes insulin-dependent, they are in stage 3 T1D.
- We got updates on several automated insulin delivery (AID), or artificial pancreas, systems, including the:
- Medtronic MiniMed 780G, especially the importance of initiating it as soon as possible following diagnosis (which is now recommended in the ADA Standards of Care for both children and adults), citing the CLVer trial, which found clinically meaningful and sustained improvements in blood sugar management following early AID initiation.
- Medtronic MiniMed 780G in high-risk youth with T1D, with 80 participants aged 7-25 years, who demonstrated an average HbA1c reduction of 2.5% (from an average baseline HbA1c of 10.5% to 8%), improvement in time-in-range, and a reduction in low blood sugar events.
- Tandem Mobi among early pediatric and adult adopters.
- Sequel Med Tech’s twist AID system, which was FDA-cleared for people with T1D aged 2+ in March 2024. The system uses the DEKA Loop algorithm, which is based on the FDA cleared Tidepool Loop (iAGC) and is intended for use with compatible interoperable continuous glucose monitors (iCGMs). Sequel is Tidepool’s first publicly announced insulin delivery device partner with an FDA-cleared system that will integrate Tidepool Loop.
- In therapy, the full INHALE-3 results demonstrated the non-inferiority of inhaled insulin (Afrezza) used with insulin degludec compared to usual care. Baseline HbA1c was 7.6% across groups, and, on average, HbA1c in both groups remained stable from baseline to 17 weeks. Overall, 30% of the inhaled insulin group reached <7.0% A1c at 17 weeks compared to only 17% of the usual care group.