The short version
Breakthrough T1D’s newest publication outlines what the future of beta cell replacement therapies looks like—and how we can make these therapies a reality for everyone with type 1 diabetes (T1D) who wants them through innovative clinical trial design and expanding the pool of eligible trial participants.
Breakthrough T1D, in collaboration with other leading experts in the field, recently published an article titled “Future Directions and Clinical Trial Considerations for Novel Islet β-Cell Replacement Therapies for Type 1 Diabetes” in the journal Diabetes. Breakthrough T1D Research and Advocacy staff who contributed to the publication include Sanjoy Dutta, Ph.D., Chief Scientific Officer, Esther Latres, Ph.D., Vice President of Research, and Marjana Marinac, Pharm.D., Associate Vice President of Regulatory Affairs.
Beta cell replacement therapies have the potential to cure type 1 diabetes (T1D) by removing the need for external insulin. While donor islet transplantation can result in insulin independence and results from early trials with manufactured islets are encouraging, people with T1D need better tools and therapies. This publication serves as a roadmap for the entire field—including researchers, product developers, industry, regulators, clinicians, and people with T1D—to address these needs and ensure that beta cell replacement therapies can get to people with T1D as quickly and safely as possible.
Read on to learn more about what the future of beta cell replacement therapies looks like.
Key Takeaways
- Currently available beta cell replacement therapies are limited to people with T1D with unstable blood sugar management, typically measured by HbA1c levels, in addition to dangerous lows (hypoglycemic events) that require immediate assistance. These therapies also require lifelong immunosuppression.
- New and improved beta cell replacement therapies are on the way, and we need to make sure that they are available for people with T1D beyond those with unstable blood sugar management and severe hypoglycemic events.
- Clinical trials for these therapies must be strategically designed to include more people with T1D given the potential for insulin independence.
- Shared decision-making between people with T1D and their care team will be critical for evaluating the risks versus benefits of a beta cell replacement therapy, especially as more options become available.
Where we are now
People living with T1D depend on external insulin to manage their condition throughout their lives. Despite advancements in diabetes technology, such as continuous glucose monitors (CGMs) and automated insulin delivery (AID) systems, most people with T1D are unable to achieve blood sugar targets, rendering them at a higher risk for complications, reduced quality of life, and lower life expectancy.
It’s simple: we need to do more for the T1D community.
One promising avenue to meet these needs is through cell replacement therapy. Lantidra®, the first FDA-approved donor-derived islet replacement therapy for T1D, has proven to be safe and effective in eliminating severe hypoglycemia, providing insulin independence, and improving quality of life. However, this therapy is limited to people with severe hypoglycemia and requires immunosuppression to prevent rejection of the transplanted cells, which can have side effects. Even more, these donor-derived islets are in limited supply.
Alternative beta cell replacement therapies are emerging—and we can no longer limit their development to people experiencing severe hypoglycemia.
As stated in the publication:
Given the proven benefits of islet transplantation extending far beyond the amelioration of severe hypoglycemia that has been documented and the understanding of the risk profile gained over the past 20 years, consideration must be given to broadening the application for islet beta cell replacement.
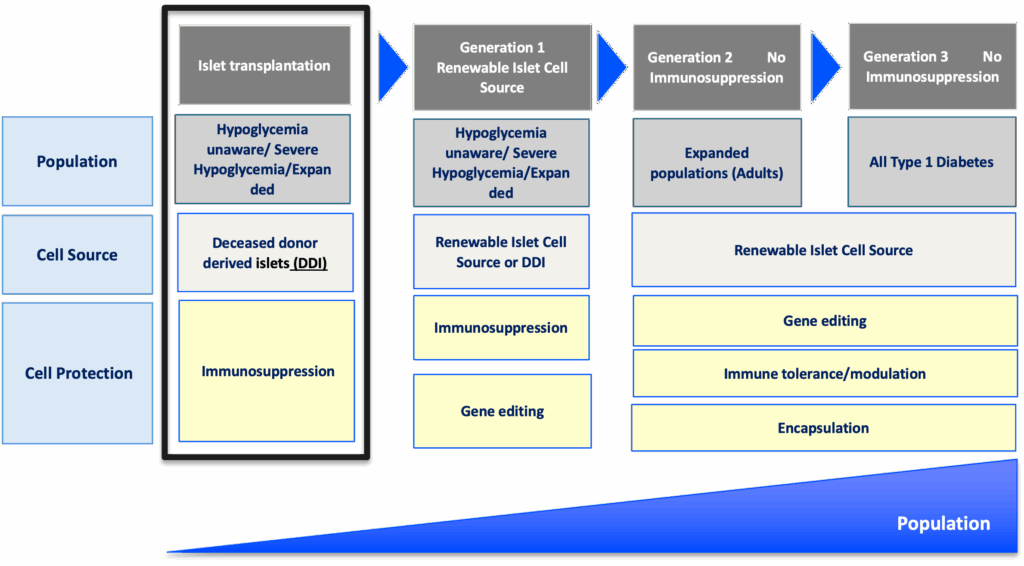
Developing cell therapy strategies to meet unmet needs of the T1D population
Breakthrough T1D’s vision for the T1D community includes beta cell replacement therapies with no immunosuppression that are available to everyone who wants them. We are committed to making this a reality through our Project ACT (Accelerate Cell Therapies) initiative, which will accelerate the development of these therapies to achieve our vision as quickly as possible.
Project ACT
Scientific progress takes time, resources, collaborations, and effort. To accelerate islet replacement therapies faster than ever, Breakthrough T1D launched Project ACT (Accelerate Cell Therapies) to simultaneously advance research, development, regulatory policies, access, and adoption of manufactured islet therapies that do not require broad immunosuppression.
We are entering an exciting era of beta cell replacement. Emerging therapies are addressing challenges such as cell source and scalability, resulting in the development of islets derived from sources other than donors, including manufactured islets and porcine islets. Up-and-coming therapies are also testing different transplantation sites, methods of delivery, and cell protection strategies to prevent immune rejection with the fewest side effects possible (and ideally no immunosuppression). Learn more about what scientists are doing to optimize beta cell replacement therapies.
Breakthrough T1D’s continuous support of many of these therapies, such as Vertex’s manufactured islet therapy zimislecel, has been critical to accelerating them through the clinical pipeline. Explore emerging beta cell replacement therapies in clinical trials now—and see how Breakthrough T1D’s commitment to these therapies helped make this possible.
When successful, the advent of new, safe, scalable, and effective beta cell replacement therapies will provide the T1D community with options. As these therapies are moving their way through the pipeline, we need to ensure they are being studied in a broader T1D population who stand to benefit.
So, how do we make this happen?
The roadmap for emerging beta cell replacement therapies
The goal
Accelerate availability of emerging next-generation beta cell replacement therapies for every person with T1D who wants them by designing clinical trials that speed their development, regulatory approval, access, and adoption.
Expanding the T1D population eligible for beta cell transplantation
Current trials testing beta cell therapies necessitating immunosuppression require participants to have elevated HbA1c levels and recurrent severe hypoglycemic events. This limits the pool of participants to people who meet the requirements and are most likely to benefit, given the side effects associated with chronic, broad immunosuppressants.
Clinical trials for emerging beta cell replacement therapies should broaden eligibility criteria so more people with T1D can participate—and experience the potential benefits. When designing new clinical trials, sponsors and regulators should consider including a broader range of HbA1c levels, clinically important or serious hypoglycemic events, and other complications.
Studies have found that the T1D community is generally open to beta cell replacement therapies as a potential solution to T1D, and people are willing to accept the associated risk versus benefit considerations for the possibility of becoming insulin independent. A Breakthrough T1D assessment also found that physicians are interested in recommending beta cell replacement therapies to people with T1D—especially if they don’t require immunosuppression.
“Clinical trials to support the development of islet cell replacement therapies need to evolve to include a broader representation of people living with T1D who could benefit from these novel therapies. This includes expanding the outcomes used to assess the benefits of cell replacement that reflect how people with T1D feel and function.”
Marjana Marinac, Pharm.D., Associate Vice President of Regulatory Affairs

Placing people with T1D at the center of clinical trial design
The outcomes used to assess the effectiveness of cell therapies currently in clinical trials, including those involving deceased donor islets, are acceptable for emerging beta cell replacement therapies. These include on-target HbA1c levels, absence of severe hypoglycemia, significant reduction or elimination of external insulin, and restoration of the body’s insulin production as measured by C-peptide.
Other endpoints that should be considered include CGM metric targets like time-in-range in addition to person-reported outcomes. Understanding how a beta cell therapy may affect a person’s health-related quality of life—such as diabetes distress, fear of hypoglycemia, or social and family dynamics—will be critically important for calculating the risk to benefit ratio of these therapies.
Read more about why person-reported outcomes are important for cell therapies.

“There are still significant unmet needs in the T1D community. Breakthrough T1D’s roadmap is supported by the assessment of clinically meaningful outcomes and driving research toward solutions that address key factors such as cell sources and protections strategies that will broaden the people with T1D who could benefit from emerging cell replacement therapies.”
Esther Latres, Ph.D., Vice President of Research
Innovative trial designs to accelerate development of cell therapies
Clinical trials for beta cell replacement therapies are generally based on a single-arm, open-label design—meaning there is no placebo group and both participants and researchers know which therapy is being administered. While this design can work for emerging beta cell therapies, single trials with multiple arms testing alternative transplant sites, immune protection strategies, or other methods have the potential to speed up the pace of development.
Similarly, adaptive trial designs use mid-trial interim analyses of study data to inform the remainder of the trial. This helps researchers learn what’s working (or not working) and adjust the design accordingly, with guidance from regulatory agencies, so the rest of the trial is a focused and efficient use of time and resources. Potential interim changes to trial design include reducing the number of participants required, eliminating doses, recruiting people who are most likely to benefit, or stopping the trial outright due to clear success or failure.
By applying guidance in therapeutic development and innovative trial designs to emerging beta cell replacement therapies, we can move early-stage trials along faster, thereby allowing regulators to make decisions sooner. To support quicker trials and reduce the possibility for delays, researchers, developers, and regulators around the world need to work together to achieve convergence on trial populations, endpoints, and innovative designs that will meet regional requirements.
Learning from past successes
People with T1D continue to live with unmet needs with still significant risk for long-term complications, and they need more therapeutic options. Right now, most clinical trials for beta cell replacement therapies requiring immunosuppression are limited to a small portion of the T1D population. This needs to change—especially given the potential for insulin independence. The T1D community must be put first when making decisions about beta cell replacement therapies, and Breakthrough T1D is making sure that this happens.
Adjusting how we approach clinical trials for emerging beta cell replacement therapies will be critical for ensuring we accelerate the research, development, regulatory approval, access and adoption of these novel therapies. Breakthrough T1D successfully accomplished this for AID systems—and we are confident that following a similar roadmap for cell therapies will get us to the finish line, faster.
The journey of AID systems
Learn more about the critical role of Breakthrough T1D in driving AID systems forward, recounted by Breakthrough T1D volunteer Doug Lowenstein.
Curative therapies for T1D are in reach. This roadmap, in conjunction with our Project ACT initiative, is key to bringing beta cell replacement therapies to every person with T1D who wants them.
To make this a reality, everyone needs to work together. As stated in the publication, “This requires a comprehensive strategy and a coordinated collaboration across stakeholders in every field relevant to islet cell replacement.” This roadmap is a guide for moving toward our common goal of a cure for T1D as soon as possible.
Breakthrough T1D will continue to lead the way until the T1D community can choose the beta cell replacement therapy that works best for them, regardless of blood glucose management or hypoglycemia status. Everyone deserves the chance to benefit.

Sana Biotechnology’s hypoimmune (HIP) donor-derived islets are genetically engineered to avoid immune destruction without the need for immunosuppressants.
Earlier this year, we reported on an update from the first person with type 1 diabetes (T1D) to receive a transplant of these cells, which showed that they were making insulin—without immunosuppression—after four weeks. Then, at the American Diabetes Association (ADA) 85th Scientific Sessions, we heard firsthand from Per-Ola Carlsson, M.D., Ph.D., the Principal Investigator at Uppsala University conducting the study, who provided more exciting data to a packed audience.
Now, these data have just been published in the prestigious New England Journal of Medicine in an article titled “Survival of Transplanted Allogenic Beta Cells with No Immunosuppression.” This study was supported by the Helmsley Charitable Trust, a long-time partner of Breakthrough T1D.
Read on to learn more.
Surviving and thriving
In this first-in-human study, the recipient of Sana’s HIP islets had no detectable insulin production at the time of the transplant (as measured by C-peptide). They received a small dose of islet cells—less than the eventual therapeutic dose—implanted into the forearm to evaluate safety.

C-peptide
C-peptide is an easily measurable biomarker of insulin production and islet function.
After 12 weeks, researchers found that the transplanted islets were successfully avoiding destruction by the immune system—meaning they are surviving AND making insulin!
The recipient had decreased HbA1c levels and stable insulin production between seven days and 12 weeks post-transplant, although, as expected with the small dose of cells used in this pilot study, they still required external insulin therapy. They experienced some non-serious side effects possibly related to the surgical transplant procedure, but not the islet cells themselves. Overall, the recipient is doing well and in good health—an exciting step for the T1D community.
Why this matters
To put it plainly: this is a huge step toward cures for T1D.
Immunosuppression is a major barrier to cell therapies because of the side effects associated with them. This is the first proof-of-concept evidence that we can genetically engineer islets to avoid immune destruction—eliminating the need for chronic, broad immunosuppressants. This means that more people may have the opportunity to benefit.
Importantly, the person in this study received only a fraction of the islet cells that would eventually be used therapeutically. Researchers are just beginning to understand the full potential of engineered HIP islets. Because more islet cells means more insulin production, there’s the possibility of insulin therapy independence if higher doses of HIP islets are implanted—something that will be addressed in future studies.
Thanks to this study, we’ve gained more evidence that forearm muscles may be a possible minimally invasive site for islet transplantation. This transplant site also provides researchers with the advantage of visualizing the islets—and making sure they’re alive—using magnetic resonance imaging (MRI), which isn’t possible with the current transplant site in the hepatic portal vein.
Excitingly, Sana is working to apply this technology to manufactured islets, opening the doors to producing HIP islets at large scale. They expect to begin phase 1 clinical trials for this next-generation HIP islet therapy as early as 2026.
Cell therapies without immunosuppression are a Breakthrough T1D priority
Cell replacement therapies—especially those that do not require immunosuppression—are a priority of Breakthrough T1D’s Cell Therapies Program and the goal of our Project ACT (Accelerate Cell Therapies) initiative.
Project ACT
Scientific progress takes time, resources, collaborations, and effort. To accelerate islet replacement therapies faster than ever, Breakthrough T1D launched Project ACT (Accelerate Cell Therapies) to simultaneously advance research, development, regulatory policies, access, and adoption of manufactured islet therapies that do not require broad immunosuppression.
To accelerate the development of cell therapies that do not require immunosuppression, The T1D Fund: A Breakthrough T1D Venture invested in Sana to help advance their HIP technology platform. Breakthrough T1D continues to support creative and promising approaches that may eliminate the need for immunosuppression with cell replacement therapies, and we are hopeful and excited about what the future may hold.
This wouldn’t have been possible without supporters like you. Thank you for all that you do to drive Breakthrough T1D’s mission, and together we celebrate each step towards a world without T1D.

ADA Recap Series
This article is the second of our three-part ADA Recap Series. Breakthrough T1D was on site in Chicago, IL from June 20-23 for the American Diabetes Association’s (ADA) 85th Scientific Sessions. We’re here to report on the latest-and-greatest type 1 diabetes (T1D) advancements—including many driven by Breakthrough T1D funding. Look out for tomorrow’s article for updates on Medical Affairs.
Cures
Breakthrough T1D’s Cures program focuses on early detection, disease-modifying therapies, and cell therapies with the goal of working toward effective cures for T1D.
Cell therapies were front-and-center at ADA 2025. We have some exciting clinical trial updates and new ideas for optimizing islet transplantation.
Cell therapies

Autologous cell transplantation
Autologous cells are those removed from an individual and implanted back into the same individual. These cells can be modified in a laboratory before implantation. Autologous cells are still susceptible to autoimmunity in T1D, so cell protection strategies (gene-editing, encapsulation, immune modulation, etc.) are expected to be required.

Allogenic cell transplantation
Allogenic cells are those that are derived from a source other than the recipient, such as deceased donors or precursor-derived manufactured cells. Allogenic cell transplants require immunosuppression because they stimulate an immune response. Breakthrough T1D’s Cell Therapies program is focused on allogenic cells—specifically manufactured cells—because they can be generated at large scale.
One-year updates on Vertex’s manufactured cell therapy, zimislecel
- Presenter: Michael Rickels, M.D. (University of Pennsylvania)
- Zimislecel (VX-880) is a manufactured islet therapy that requires immunosuppression, infused into a vein in the liver in people with T1D who have impaired hypoglycemic awareness and severe hypoglycemic events.
- The phase 1/2 clinical trial, which is part of the pivotal phase 1/2/3 FORWARD-101 trial, is complete. Twelve participants received a single infusion of a full dose of cells and were followed for at least one year.
- All 12 participants achieved the primary endpoint, which was elimination of severe hypoglycemic events and HbA1c levels less than 7%. 10/12 (83%) participants are insulin independent.
- All 12 participants demonstrated sustained insulin production as measured by C-peptide, reduced external insulin therapy use, and achieved greater than 70% time in range.
- There were no serious adverse events. Mild to moderate adverse events were consistent with the immunosuppression regimen, infusion procedure, and complications from T1D.
- These data were published in the New England Journal of Medicine and represent further evidence of the curative potential of manufactured islet transplantation for T1D.
- Breakthrough T1D’s support for Doug Melton, Ph.D.—whose proprietary lab-created beta cells are now being advanced by Vertex—goes back decades, both via research grants and an investment from the T1D Fund: A Breakthrough T1D Venture.
6-month update on Sana Biotechnology’s immune-evasive islets
- Presenter: Per-Ola Carlsson, M.D., Ph.D. (Uppsala University)
- Sana’s donor-derived islet therapy engineered with Hypoimmune (HIP) technology can evade the immune system without immunosuppression.
- These cells were implanted intramuscularly in a first-in-human study into a person with T1D with no measurable insulin production.
- Six months post-transplant, this person is consistently making their own insulin, as measured by C-peptide levels. Yet, they still require external insulin therapy because they received a smaller dose of cells than the dose that would be required to achieve insulin independence. They did not experience any serious side effects, so the cells and procedure are safe and well-tolerated.
- A Mixed Meal Tolerance Test (MMTT) confirmed that these cells are not only surviving but also responding to changes in blood glucose levels.
- This is a promising first step toward a functional cure for T1D that does not require immunosuppression. Sana Biotechnology is planning on applying this technology to manufactured islets.
- Sana has received support from the T1D Fund to advance their HIP technology in islets, and Breakthrough T1D continues to work closely with them.
A new transplantation site for autologous manufactured islets
- Presenter: Hongkui Deng, M.D. (Peking University)
- Cells derived from adipose tissue (fat) can be removed from a person and chemically induced in the laboratory to become islet cells.
- Implantation of autologous manufactured islets into the sub-anterior rectus sheath in preclinical models of T1D improves glycemic control.
- In humans, this implantation site is easily accessible by an ultrasound-guided needle.
- In a first-in-human study, autologous manufactured islets were implanted into this site in a person with T1D. This person no longer needs external insulin therapy and has greatly improved blood glucose control. This person had also received a liver transplant and was taking immunosuppressants.
A new encapsulation device for immune protection of transplanted islets
- Presenter: Nicolas Laurent, Ph.D. (Adocia)
- Adoshell® is a novel islet cell encapsulation device that can shield islets from the immune system, meaning that immunosuppressants are not needed.
- The hydrogel-based device is non-degradable, easily retrievable, and allows the exchange of glucose and insulin from the vasculature surrounding the device while excluding immune cells from encapsulated islets based on pore size.
- This device showed promise in animal models, and human clinical testing is next.

Cell therapies highlight: Breakthrough T1D-funded research
Tom Bollenbach, Ph.D. (Advanced Regenerative Manufacturing Institute; ARMI) presented on challenges and solutions for large-scale manufacturing of islet cells. The goal is to generate scalable, automated manufactured islets that can be used by labs around the world to accelerate research progress using a unique, validated, and reliable cell source. Some challenges include ensuring the manufactured cells can survive shipment from the facility to research labs and maintain their insulin-producing capacity when they are used in different labs. ARMI is working with the Beta Cell Replacement Consortium to address these challenges.
Antonio Citro, Ph.D. (San Raffaele Hospital) presented his work on ensuring that transplanted islets have enough oxygen and nutrients (“vascularization”) to survive and function. Dr. Citro described an approach tested in animal models referred to as “natural scaffolds” in which all cells are removed from a donor organ, such as a lung, leaving behind blood vessel structures and other structural components. Islet cells can be injected and grafted onto this scaffold to create a mini organ of islet cells, which can then be tested for functionality before and after implantation.
Andrew Pepper, Ph.D. (University of Alberta) also presented vascularization strategies to increase the survival and functionality of transplanted islets. The implantation of a biomaterial under the skin will trigger the immune system and initiate a foreign body reaction, which results in the formation of blood vessels and structural components around the foreign object. Removal of the object leaves a hollow pre-vascularized core suitable for islet transplantation. This process can be optimized by using a biodegradable material, so no removal is required, and by the addition of “accessory cells” that help maintain a vascularized environment for islets.
Key takeaways
Cell therapies are making significant headway in clinical trials, and people receiving manufactured cells are becoming insulin independent. Researchers are tackling the biggest challenges for optimizing islet transplantation, including large-scale manufacturing, ensuring cell survival, and preventing detection by the immune system.
Disease-modifying therapies
A major focus at ADA 2025 was addressing the underlying immune mechanisms of T1D—including alterations in immune cells that facilitate beta cell destruction and other factors that contribute to autoimmunity onset. Read on for some highlights.
The role of B cells in T1D autoimmunity
- Presenter: Mia Smith, Ph.D., DVM (University of Colorado)
- B cells are a type of immune cell that can activate destructive immune cells that facilitate autoimmunity in T1D.
- B cells can become wrongly activated against insulin-producing beta cells due to converging dysregulation of factors that regulate immunity.
- These cells represent another potential target for disease-modifying therapies in T1D.
Disease-modifying therapies highlight: Breakthrough T1D-funded research
Emrah Altindis, Ph.D. (Boston College) presented on the role of the gut microbiome in T1D. His studies found that people with T1D tend to have more inflammatory bacteria in their gut microbiome. Dr. Altindis and his team identified a particular bacterial population that can enhance T1D onset in animal models due to changes in immune cells that contribute to T1D autoimmunity. These studies provide insight into additional factors that can drive immune changes in T1D.
Laura Sanz Villanueva, MSc (St. Vincent’s Institute of Medical Research), who works in the lab of Breakthrough T1D-funded researcher Professor Thomas Kay, MBBS, Ph.D., presented on a mechanistic follow-up study to the BANDIT clinical trial. The Breakthrough T1D-funded phase 2 BANDIT study showed that baricitinib, a JAK1/2 inhibitor that prevents immune cell communication, can increase insulin production as measured by C-peptide in people with recently diagnosed T1D. The present study found that baricitinib can reduce the number of natural killer (NK) cells in the pancreas, which are involved in the autoimmune destruction of beta cells. These data provide valuable insight into the mechanism of baricitinib-mediated protection of beta cells.
Key takeaways
T1D is driven by dysregulation of the immune system, which results in an attack on insulin-producing beta cells. Researchers at ADA 2025 spoked about novel factors—including B cells, NK cells, and T1D autoimmunity triggers—that may contribute to immune cell dysfunction in T1D.
Early detection
A key focus at ADA 2025 was the growing recognition of the heterogeneity of T1D, including autoantibody-negative disease onset, genetic variation, and the frequent misdiagnosis of T1D in adults, underscoring the need for greater diversity and inclusion in research and care. The expanded role of continuous glucose monitoring (CGM) and continuous ketone monitoring (CKM) was also highlighted, not only for daily management but as essential tools for understanding disease progression.
Using genetics to predict T1D risk
- Presenters: Richard Oram, M.D., Ph.D. (University of Exeter), Leslie Lange, Ph.D. (University of Colorado), Aaron Deutsch, M.D. (Massachusetts General Hospital), Josep Mercader, Ph.D.(Massachusetts General Hospital) and Eimear Kenny, Ph.D. (Icahn School of Medicine at Mount Sinai)
- Polygenic risk scores (PRS) estimate the risk a person has for developing a disease like T1D based on variations in different genes.
- Ancestry is a major influence on PRS, particularly based on differences in genes that regulate whether the immune system can distinguish between “self” and “non-self.”
- Most PRS models have been developed using data from European populations and have a limited ability to accurately determine risk in other ethnic groups, such as individuals of African and East Asian descent.
- Potential applications of PRS include incorporation into screening to better understand T1D risk, ensure accuracy in diagnostic tests, and develop precision medicine-based therapeutic approaches.
Understanding how genetic diversity contributes to T1D
- Presenters: Suna Onengut-Gumuscu, Ph.D. (University of Virginia), Dominika A. Michalek, MS (University of Virginia), Aaron Deutsch, M.D. (Massachusetts General Hospital), and Stephen I Stone, M.D. (Washington University School of Medicine), among others.
- These talks highlighted several studies conducted in diverse populations to better understand the pathophysiology of T1D.
- Work presented from Consortia, such as RADIANT, focused on rare and atypical forms of diabetes.
Controversies in CGM and benefits for early detection
- Presenters: Peter Calhoun, Ph.D. (Jaeb Center for Health Research), Michael Kohn, M.D., MPP (University of California San Francisco), Nicole Ehrhardt, M.D. (University of Washington) and Tadej Battelino, M.D., Ph.D. (University of Ljubljana)
- CGM use holds value in identifying progression in early stages of T1D prior to symptomatic onset.
- There was a call to update the clinical guidelines so that the benefits of CGM can be maximized within the T1D community—including at early and later stages of T1D.
- Integrating newer measures of blood glucose, like the glucose management indicator (GMI) and time in tight range (TITR), will be essential.
Contributions of CKM to early detection
- Presenters: Ketan Dhatariya, MBBS, M.D., Ph.D. (Norfolk and Norwich University Hospitals), Lori Laffel, M.D., MPH (Harvard University), Jennifer Sherr, M.D., Ph.D. (Yale University), and Richard Bergenstal, M.D. (HealthPartners Institute).
- It will be critical to explore whether ketone monitoring could help reduce the incidence of diabetic ketoacidosis (DKA) at stage 3 clinical T1D onset.
- Early detection of rising ketones will be important for people with T1D to take action before DKA occurs.
Early detection highlight: Breakthrough T1D-funded research and awardees
Brigitte Frohnert, M.D., Ph.D. (Barbara Davis Center for Diabetes), co-investigator with Breakthrough T1D-funded researcher Andrea Steck, M.D., presented on the evolution of CGM patterns prior to stage 3 T1D. Their longitudinal approach, with CGM data collected at three-month intervals, successfully distinguished individuals who progressed to stage 3 T1D clinical onset from those who did not. This research suggests that CGM may be beneficial for predicting clinical progression of T1D.
Anette-Gabriele Ziegler, M.D. (Helmholtz Munich) was awarded the Harold Hamm Prize for Biomedical Research in Diabetes 2025, highlighting her important contributions on T1D screening in the pediatric population.
Barbara B Kahn, M.D. (Beth Israel Deaconess Medical Center and Harvard Medical School) received the 2025 Albert Renold Award, and her role model for women in science was highlighted at the Women’s Interprofessional Network of the ADA (WIN ADA).
Key takeaways
T1D arises in diverse ways across age groups and ancestries, complicating diagnosis and treatment. At ADA 2025, experts highlighted how genetic and clinical heterogeneity demands more inclusive strategies. Tools like CGM and CKM were highlighted for their potential to enhance clinical management in the early stages of T1D.

Breakthrough T1D’s Cures team making an impact
Sanjoy Dutta, Ph.D., Chief Scientific Officer, participated in a panel discussion titled “Encapsulation vs. Naked Cell Therapy—Immune Challenges and Beta-Cell Perspectives in Diabetes Treatment.” The panelists covered different approaches to preventing immune attack of transplanted islets, including gene-editing, in addition to pros and cons of different transplantation sites.
Esther Latres, Ph.D., Vice President of Research, and Jay Tinklepaugh, Ph.D., Senior Scientist, hosted a workshop immediately after ADA titled “Islet Cells in T1D.” Breakthrough T1D-funded researchers gathered from around the world to discuss cell therapy clinical trial updates, mechanistic insights into islet function, targeted delivery of therapeutics to beta cells, and optimization strategies for islet transplantation.
Look out for tomorrow’s article for an update on Medical Affairs presented at ADA 2025!

ADA Recap Series
This article is the first of our three-part ADA Recap Series. Breakthrough T1D was on site in Chicago, IL from June 20-23 for the American Diabetes Association’s (ADA) 85th Scientific Sessions. We’re here to report on the latest-and-greatest type 1 diabetes (T1D) advancements—including many driven by Breakthrough T1D funding. Look out for tomorrow’s article for updates on Cures.
Improving Lives
Breakthrough T1D’s Improving Lives program focuses on devices, insulins, adjunctive therapies, treatments for complications, and psychosocial interventions to improve the health and quality of life of people living with T1D.
Adjunctive therapies and complications
There was significant focus on GLP-1 receptor agonists (GLP-1RAs) and SGLT inhibitors (SGLTi) in reducing long-term complications and improving glycemic control in people with T1D.

GLP-1 receptor agonists
Glucagon-like peptide 1 receptor agonists mimic the hormone GLP-1, which elevates insulin and regulates appetite. Examples include Ozempic® (semaglutide) and Mounjaro® (tirzepatide), which acts on both GLP-1 and a similar target, GIP.

SGLT inhibitors
Sodium-glucose cotransporter inhibitors target kidney cells to prevent them from reabsorbing glucose into the blood so it gets excreted as waste. Examples include Farxiga® and Zynquista®.
While SGLTi and GLP1-1RAs have proven effective for heart and kidney disease in type 2 diabetes (T2D) and in people without diabetes, people with T1D have often been excluded from critical trials. Thanks to years of advocacy and support from Breakthrough T1D, T1D trials are ongoing—and real-world evidence suggests that GLP-1RAs and SGLTi could be impactful in the T1D community as well.
Real-world evidence for GLP-1RA use in T1D
- Presenter: Ildiko Lingvay, M.D.; University of Texas Southwestern
- People with T1D have self-reported that they decided to try GLP-1RAs for weight loss and improved glycemic control.
- Real-world evidence suggests that GLP-1RAs have a clinically meaningful impact on weight and reduced insulin dose.
- While GLP-1RAs are generally safe, some people have stopped use because of gastrointestinal side effects. These side effects are also seen in people with T2D and people without diabetes.
A review of SGLTi and GLP-1RAs in reducing chronic kidney disease (CKD) in T1D
- Presenter: David Cherney, Ph.D.; University of Toronto
- In the EMPA-KIDNEY trial that included non-diabetes participants and people with T1D or T2D, empagliflozin (SGLTi) improved kidney health in people with T1D.
- In the ATTEMPT trial, dapagliflozin (SGLTi) improved time in range (TIR), reduced HbA1c levels, and had positive effects on kidneys in youth with T1D.
- The Breakthrough T1D-funded enrolling phase 3 SUGARNSALT trial is testing whether sotagliflozin (SGLTi) can prevent progression of moderate to severe kidney disease in people with T1D, and it includes careful diabetic ketoacidosis (DKA) risk mitigation strategies.
- The SEMA-AP trial found that semaglutide (GLP-1RA) increases TIR in people with T1D when used alongside an AID system.
- The Breakthrough T1D-funded recruiting phase 2 REMODEL-T1D trial is testing if semaglutide (GLP-1RA) can improve kidney health in people with T1D.

Glucokinase
Glucokinase (GK) is an enzyme in liver cells that works in an insulin-dependent manner to regulate blood sugar. In people with T1D who have little insulin reaching the liver, GK can’t work as normal, contributing to higher blood sugar.
Use of a glucokinase activator for glycemic control
- Presenter: Klara Klein, M.D., Ph.D.; University of North Carolina
- In the phase 1/2 SimpliciT1 study, people with T1D who received the GK activator TTP399 showed improvements in blood glucose with fewer hypoglycemic events.
- A different study found that TTP399 does not increase the risk for DKA.
- These studies were done in collaboration with vTv Therapeutics, a company with funding and support from Breakthrough T1D and the T1D Fund: A Breakthrough T1D Venture. The phase 3 CATT1 study for TTP399 is testing whether it can reduce moderate to severe hypoglycemic events in people with T1D.
Adjunctive therapies and complications highlight: Breakthrough T1D-funded research
Halis Kaan Akturk, M.D. (University of Colorado), Janet Snell-Bergeon, Ph.D., MPH (University of Colorado), and Viral Shah, M.D. (Indiana University) presented findings from the Breakthrough T1D-funded ADJUST-T1D clinical trial, which was recently published in the New England Journal of Medicine Evidence. The trial tested whether semaglutide (GLP-1RA) can improve glycemic and weight outcomes in adults with T1D and obesity who are using an AID system. 36% of people treated with semaglutide met the primary endpoints of TIR greater than 70%, time below range less than 4%, and weight loss of 5% or more compared to the placebo, and the drug was well-tolerated and safe. This trial represents critical evidence for use of a GLP-1RA as a potential way to manage both glycemic control and weight in people with T1D.
Ye Je Choi, MPH (University of Washington) reported on the CROCODILE study, which examined metabolic alterations in kidneys of people with T1D. She observed early structural and metabolic changes in kidneys that occurred before the onset of clinical kidney disease and associated structural damage. Her work could contribute to the development of biomarkers that can help predict the onset of kidney disease in people with T1D before it occurs.
Jeremy Pettus, M.D. (University of California at San Diego) conducted a phase 2 clinical trial to address insulin resistance in people with T1D. External insulin therapy can increase levels of insulin in the blood relative to glucose, which reduces sensitivity and may contribute to cardiovascular disease (CVD). Treatment with the glucagon receptor antagonist volagidemab, which prevents the liver from releasing glucose into the blood, reduces insulin requirements by 15%, resulting in improved glycemic control and insulin sensitivity without changes in bodyweight.
Schafer Boeder, M.D. (University of California at San Diego) worked with Dr. Pettus on a phase 1/2 clinical trial that tested whether the addition of SGLTi to the glucagon receptor antagonist volagidemab can further improve glycemic control in people with T1D. The combination of therapies increased TIR up to 86% from 70% and reduced daily insulin use by 27%. Further research is needed to better understand the safety profile of this regimen.
Justin Gregory, M.D. (Vanderbilt University) worked with Dr. Pettus and Dr. Boeder on the above study. He also presented on the use of GLP-1RAs and dual GLP-1/GIP receptor agonists for reducing complications in T1D.
Key takeaways
Clinical trials with GLP-1RAs and SGLTi are providing encouraging evidence about these therapies’ potential to improve long-term health in people with T1D. Breakthrough T1D is working toward a future where these drugs are an option for people with T1D to better manage their blood sugar and reduce complications.
Devices
Real-world insights from Automated Insulin Delivery (AID) systems
- Presenter: David Maahs, M.D., Ph.D.; Stanford University
- Based on published real-world evidence for AID systems in people with T1D, TIR is increased by an average of eight to 15% from baseline in a range of studies across various systems.
- Youth with T1D have better glycemic control and reduced rates of DKA with AID systems. Those with lower TIR at the start of AID system use see the greatest improvements.
Real-world evidence: iLet Bionic Pancreas AID system
- Presenter: Steven Russell, M.D., Ph.D.; Beta Bionics
- The iLet Bionic Pancreas contains a continuously adapting algorithm that automatically determines insulin doses. No carbohydrate counting is required, and meals are only announced as breakfast, lunch, and dinner.
- Data was collected from 16,000 users over two years.
- Users achieved an average HbA1c level of 7.3%, down from 8.9%. This is accompanied by low rates of hypoglycemia and significantly improved self-reported quality of life.
Continuous ketone monitoring: Innovations and clinical applications
- Presenters: Ketan Dhatariya, MBBS, M.D., Ph.D. (Norfolk and Norwich University Hospitals), Lori Laffel, M.D., MPH (Harvard University), Jennifer Sherr, M.D., Ph.D. (Yale University), and Richard Bergenstal, M.D. (HealthPartners Institute).
- DKA rates are increasing in the U.S., but mortality rates from DKA are decreasing.
- The history of continuous glucose monitoring (CGM) offers a roadmap for continuous ketone monitoring (CKM) development, showing how early skepticism gave way to broad clinical impact.
- CKM could allow for earlier detection of rising ketones to prevent DKA. CKM also has the potential to identity infusion set failures, be a valuable addition to AID systems, help monitor early-stage T1D, and more.
- Five new studies funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) will develop CKM for safe and effective use of SGLTi in T1D.
- Tandem, Beta Bionics, Sequel MedTech, and Ypsomed announced plans to integrate Abbott’s dual glucose ketone sensor into their AID systems.
Making the case for time in tight range
- Presenter: Gregory Forlenza, M.D.; University of Colorado
- Dr. Forlenza presented on the benefits and challenges of time in tight range (TITR), also known as time in normal glycemia (TING), defined as blood glucose levels between 70-140 mg/dL.
- TITR will likely be more clinically beneficial than TIR as fluctuations outside of TITR may be better predictors of complications and offer a better therapeutic window for intervention.
- More research is needed to advance therapeutics that will allow people with T1D to achieve TITR before it can be integrated into clinical decisions.
Devices highlight: Breakthrough T1D-funded research
Erin Cobry, M.D. (University of Colorado) presented the results of a Breakthrough T1D-funded clinical trial evaluating an artificial intelligence-powered AID algorithm designed to not require meal announcements. She showed that this algorithm (used without meal announcements) improved overnight TIR, and provided equivalent daytime TIR, compared to participants’ usual care. A major goal for Breakthrough T1D is to advance AID systems that do not require meal announcements to improve both glucose outcomes and quality of life for people with T1D.
Key takeaways
Devices have transformed how this disease is managed. Systems are becoming easier to use with less user input—and, critically, people with T1D are doing better. This is the dream Breakthrough T1D had when we launched the Artificial Pancreas project 20 years ago. We will continue to drive toward our goal of developing systems that provide superior health outcomes with minimal user burden.
Insulins
Inhaled insulin treatment for youth with T1D
- Presenter: Michael Haller, M.D.; University of Florida
- Afrezza® is an inhaled, fast-acting insulin that has proven to be effective in adults.
- The phase 3 INHALE-1 study examined Afrezza® in youth with T1D. Users report greater treatment satisfaction and no increase in weight compared to injected rapid-acting insulin analogs.
- Afrezza® is safe for youth with T1D. The most common adverse events were pulmonary-related, such as coughing.

Breakthrough T1D’s Improving Lives team making an impact
Courtney Ackeifi, Ph.D., Senior Scientist, hosted an Improving Lives Happy Hour with Breakthrough T1D-funded researcher Jeremy Pettus, M.D. The discussion included research priorities for adjunctive (non-insulin) therapies for people with T1D and their healthcare providers. They also discussed the importance of industry partnerships and the role of Breakthrough T1D in driving these relationships, which can accelerate new T1D therapies toward the clinic.
Dr. Ackeifi also spoke at the ADJUST-T1D trial update, contextualizing the use of adjunctive therapies like GLP-1RAs for superior glucometabolic control in people with T1D.
Look out for tomorrow’s article for an update on Cures research presented at ADA 2025!
Clinical trials are scientific studies that tell us if a new therapy or device works in humans and is safe. All new medical products must go through this process. Clinical trials are a necessary part of getting promising treatments from the lab and into clinics, so people can benefit—including those with type 1 diabetes (T1D).
The caveat: clinical trials don’t always work as expected for everyone. People with T1D might get the placebo instead of the actual treatment. Or, they might get the treatment, but it may not be effective for them. While this can be disappointing, it’s important to remember that clinical trials are an essential part of the process for arriving at new, effective therapies—and we can learn from them at every step of the way.
This Clinical Trials Awareness Day, we’re debunking some myths and misconceptions about clinical trials. What do researchers do when they don’t work? Why are placebos necessary? Why do they take so long?
Read on to find out.
When clinical trials “don’t work”

Assumption
A negative clinical trial—one that doesn't meet its primary endpoint or that yields inconclusive data—is a waste of time and money.

Reality
All data is good data, and we can learn lessons from clinical trials to design better studies that give us more information.
Clinical trials don’t always work the way researchers hope they will. For example, in T1D, a new therapy may not lower blood glucose, or it may increase the risk of severe hypoglycemic events. If the data from the trial isn’t positive, was it all a waste of time?
The answer is: absolutely not! The scientific method involves conducting an experiment or trial, analyzing the data, and using that data to figure out the next steps. Sometimes, researchers conclude that a drug might work better for a different indication. Other times, the trial design needs to be adjusted to make sure the right outcomes are being measured or the risk for serious side effects is reduced. Scientists and clinicians essentially use “negative” trial data to rule out what doesn’t work and craft a more informed plan going forward.
“The value of a clinical trial goes far beyond whether the drug ‘works.’ We can learn critical information from mechanistic studies that allow us to fine-tune our approaches, implement personalized and precision medicine, and ultimately improve outcomes for everyone living with T1D,” explained Joshua Vieth, Ph.D., Senior Director of Research at Breakthrough T1D. Here are some case studies that help explain this idea.
Diamyd® is a disease-modifying therapy that works by stopping the autoimmune destruction of insulin-producing beta cells. After demonstrating safety, Diamyd Medical initiated a phase 2 clinical trial for Diamyd® in Europe (DIAGNODE-2) in 2017. Initial readouts suggested that Diamyd® might be working—some people had lower blood sugar. However, at the end of the trial, there was not a clinically meaningful difference in C-peptide, a biomarker for insulin production.
The investigators didn’t give up. After taking a closer look at the data, they noticed that a portion of people achieved superior glycemic control compared to others. Going a step further, they found that the “responders” had a specific genetic marker (HLA DR3-DQ2). This marker is a risk factor for T1D that about 40% of the T1D population has.
Armed with this new knowledge, Diamyd Medical initiated a Breakthrough T1D-funded, global phase 3 trial for Diamyd® (DIAGNODE-3), targeted towards young adults and teens with the “responder” genetic marker and early stage T1D. The trial is currently progressing as planned.
Had the clinical researchers not done what they do best—ask questions and look for answers—Diamyd® would likely not have continued through the clinical pipeline.
Regulatory T cells help suppress immune responses. The Sanford Project T-Rex Trial, initiated in 2016, examined whether taking a person’s regulatory T cells, expanding them outside of the body, and putting them back in could help curb the autoimmune attack on beta cells. This was a phase 2 study in children eight to 17 years old with recent T1D onset.
In the end, the trial did not meet its primary endpoint as measured by C-peptide. Similar to the Diamyd® study, the researchers weren’t discouraged by this. They noticed that different people’s regulatory T cells grew at different rates outside of the body. Wondering why, they performed a genetic analysis of the cells and found something intriguing: regulatory T cells that grew more slowly correlated with better C-peptide when put back into the body.
Researchers concluded that it wasn’t the number of regulatory T cells that mattered, but rather the “quality.” This critical finding spurred follow-up investigations into designing new and innovative drugs that target these cells, pre-screening people to identify who might benefit from them, and incorporating mechanistic biological studies like this into clinical trial design.
SGLT (sodium-glucose cotransporter) is a protein that helps cells in the small intestine and kidney take up glucose from the blood. SGLT inhibitors that target kidney cells prevent them from absorbing glucose from the blood, so it gets excreted as waste. This class of drugs has shown benefits for glycemic control and reduced risk of complications in T2D, and researchers predicted that people with T1D could experience similar benefits.
In 2015, Lexicon Pharmaceuticals initiated three phase 3 clinical trials (inTandem1, inTandem2, and inTandem3) investigating the use of the SGLT inhibitor sotagliflozin (Zynquista™) in people with T1D and chronic kidney disease (CKD) as an adjunct therapy to insulin for blood glucose control. The results revealed clinically meaningful reductions in HbA1c—however, this was associated with a considerable risk for diabetic ketoacidosis (DKA). Lexicon tried their hand and submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in 2024. In the end, the FDA rejected sotagliflozin for people with T1D and CKD, and Lexicon shut down their T1D program—a disappointing loss for the T1D community.
As these events were unfolding, researchers saw an opportunity: reduce the risk for DKA so people with T1D and CKD can experience the benefits of glucose control with sotagliflozin. In 2019, Breakthrough T1D helped publish international consensus guidelines for DKA risk mitigation for people with T1D treated with SGLT inhibitors. New SLGT inhibitor trials began to emerge with specific DKA risk reduction strategies, such as device-based ketone monitoring. This includes SUGARNSALT: a phase 3, Breakthrough T1D-funded trial that is currently enrolling (and one of many SLGT inhibitor trials that Breakthrough T1D is supporting).
These newer trials are paving the way for safe use of SGLT inhibitors in T1D to reduce kidney complications and improve blood glucose. Soon-to-be advancements in ketone monitoring, such as continuous ketone monitor (CKM) integration with continuous glucose monitors (CGMs) and consensus guidelines, will help people with T1D feel safe and confident using this class of drugs.
GLP-1 (glucagon-like peptide 1) is a naturally occurring hormone that the body releases in the gut after eating. It stimulates insulin production, blocks glucagon, reduces blood sugar, and induces the sensation of feeling full. GLP-1 receptor agonists mimic these effects and have shown incredible promise in T2D. One such example is liraglutide (Victoza®).
In 2013-2014, Novo Nordisk initiated two phase 3 clinical trials (ADJUNCT ONE and ADJUNCT TWO) for use of liraglutide as an adjunct therapy to insulin for people with T1D to lower blood sugar. Liraglutide improved HbA1c and bodyweight but also caused an increased rate of symptomatic hypoglycemia at higher doses. Based on these results, the company decided to terminate their pursuit of this therapy for T1D and did not submit an application to the FDA.
Knowing the potential benefits of GLP-1 receptor agonists for people with T1D, Breakthrough T1D continued to invest in this space to gather more clinical data and optimize trial design. This includes strategies for hypoglycemia risk reduction, especially since this class of drugs can increase insulin sensitivity.
Lo and behold, Eli Lilly recently initiated a recruiting phase 3 clinical trial (SURPASS-T1D-1) that will test whether tirzepatide (Mounjaro® or Zepbound®), a dual GLP-1/GIP receptor agonist, can improve blood glucose control in people with T1D who are obese or overweight, alongside insulin therapy. This is the first industry-sponsored clinical trial for tirzepatide in T1D.
While there are currently no GLP-1 receptor agonists approved for T1D, it’s hugely encouraging that companies are investing in this space. Between this and several Breakthrough T1D-funded trials, we are sure to see the future of GLP-1 receptor agonists for glucose control in T1D sooner rather than later.
The low-down on placebos

Assumption
It’s not fair for people with T1D to get a placebo instead of the study drug since it doesn’t do anything.

Reality
Placebos are required to know if a new therapy is working, and by participating in clinical trials you will receive top-tier care and contribute to medical breakthroughs that could help the entire T1D community.
Placebo
A placebo is a substance or procedure that does not contain any active ingredients and has no therapeutic effect. It usually looks similar and is given the same way as the therapy that it is being tested against.
Placebos are a necessary part of ethical, scientifically rigorous clinical trial design. Without them, researchers wouldn’t be able to tell if a new therapy has a clinically meaningful effect or not.
An important aspect of placebo-controlled trials is “blinding” participants, and usually clinicians, from knowing if they are giving or receiving the study drug or a placebo. This accounts for the placebo effect, which occurs when a person feels better, even though they received a placebo, because they believe that it’s having an effect. By separating the placebo effect from the actual biological effects of a new therapy, researchers can draw stronger conclusions about whether it will have a clinical benefit.
Clinical trials, especially at earlier stages, are also designed to test safety. A phase 1 trial is usually the first time that a new therapy is being put into humans. Without a placebo group, researchers wouldn’t have a clear picture of any side effects that a new treatment may have—which is critical for advancing to the next trial phases.
Clinical trials must be designed ethically: prioritizing safety, transparency, and clinical value. Placebos are a part of this, and they shouldn’t be a deterrent to enrolling in trials. People in the placebo group get state-of-the-art clinical care and are contributing to the body of knowledge that helps researchers and regulators make the right decisions about new therapies—which at the end of the day, benefits the entire T1D community.
The waiting game

Assumption
Clinical trials take too long and as such are not worth supporting.

Reality
Clinical trials take a long time because they thoroughly evaluate if a new therapy is safe and has a clinical effect before being available to everyone.
Clinical trials require coordination between lots of people: identifying study sites, making sure the clinics have the right personnel to administer a new treatment, making enough high-quality drug to conduct trials, and recruiting eligible participants. This also includes communication with regulatory agencies and extensive paperwork, data review and analysis by scientists and clinicians, writing up and publishing results, and ethics reviews.
It’s true: clinical trials take a long time. But, it’s all in the name of prioritizing people’s health, and most would agree that getting a new therapy that hasn’t been fully vetted wouldn’t be the best idea. Often, recruiting enough people is a major barrier to the advancement of trials. While many members of the T1D community participate in clinical trials, we need more people to accelerate the pace.
Conclusion: Clinical trials are thoughtfully designed—and essential to our health

Reality
Without clinical trials, we wouldn’t have access to the life-changing therapies that are available today.
Money, time, and energy spent on clinical trials is well worth the investment. Clinical trials, in essence, are the scientific process of making sure that a new therapy is safe and effective before it gets to people with T1D. Researchers learn from unexpected results to better decide on next steps, and each aspect of trial design is well thought out and has a specific purpose. While they often take many years, in the end it’s worth it.
In short: trust the process!

“Taking part in a clinical trial is such an important contribution to the T1D field. Beyond determining whether the treatment works, we are learning critical information about how the cells of the body respond. This opens the door for next generation approaches and allows us to take a giant step forward in the development of treatments and cures. Your participation in a trial provides long-term benefits to all those currently living with, or at risk of developing T1D.”
Joshua Vieth, Ph.D., Senior Director of Research at Breakthrough T1D
Participate in clinical trials
Contribute to scientific advancements and drive T1D breakthroughs by participating in clinical trials. With your help, we can move clinical trials along faster—and get the latest and greatest T1D therapies to people sooner!

Project ACT series
This article is part of a series exploring the different ways that Breakthrough T1D’s Project ACT (Accelerate Cell Therapies) is shaping the future of cell therapies for type 1 diabetes (T1D). The next article in the series will focus on Project ACT’s advocacy efforts to ensure there is a regulatory pathway to approval for these therapies and that they will be covered by payers.
Read last month’s article about challenges and solutions of T1D cell therapies.
Despite significant advances in treatments for T1D, our community still has significant unmet needs. Breakthrough T1D believes that novel cell therapies will transform T1D management, and Project ACT is how we’re going to make them a reality.
First-generation cell therapies, including FDA-approved, donor-derived Lantidra® and Vertex’s manufactured islet therapy in phase 1/2/3 clinical trials, VX-880 (Zimislecel), are incredibly promising. They have some limitations, including:
- There are not enough donor-derived islets to meet the needs of everyone with T1D.
- These therapies are only available to people with severe hypoglycemia unawareness and hypoglycemic events.
- The number of people who can receive these therapies is further limited in that they must be able to tolerate chronic, broad immunosuppression.
Current research efforts at the preclinical, clinical, and manufacturing levels are working to address these challenges. The ultimate goal is a future in which manufactured islet therapies exist in large supply, survive and produce insulin in the body after implantation, and remain protected from the immune system. Learn more about Breakthrough T1D’s Cell Therapies Program and take a closer look at what researchers are doing to turn these ideas into a reality.
Clinical trials to keep an eye on
Up-and-coming cell therapies for T1D are in the clinical pipeline and working their way towards the market, including many that Breakthrough T1D has contributed to. There are some highly anticipated (and currently enrolling!) trials that we have our eyes on right now, and we hope to see data soon. Read about each study in detail below or scroll down to see a summary table.
Late last year, Vertex announced the expansion of their manufactured islet therapy, VX-880 (Zimislecel), to a phase 1/2/3 clinical trial, the final step before seeking FDA approval. This decision stemmed from groundbreaking data in the initial phases of the trial in which 11 of 12 participants reduced or eliminated the need for external insulin therapy. Currently, zimislecel is limited to people with severe hypoglycemia and requires chronic immunosuppression.
The results of the VX-880 trial are highly anticipated since this is the first time a scalable treatment for T1D has entered a final clinical testing stage, and regulatory submission is expected in 2026. Vertex is working closely with regulators to expand its manufacturing capabilities and ensure they are prepared for the therapy to hit the market.
Zimislecel would not have been possible without years of support from Breakthrough T1D and The T1D Fund: A Breakthrough T1D Venture. This includes research grants, an investment by the Fund in Semma Therapeutics (which was later acquired by Vertex), and much more.
It doesn’t stop there: Vertex is expanding their pipeline and investigating different ways to keep manufactured islets safe without standard anti-rejection immunosuppressants, including alternative immunosuppressive regimens, gene-edited immune-protected cells, and novel encapsulation devices.
Although Vertex’s T1D portfolio is progressing, the clinical development of VX-264, an encapsulated islet therapy that does not require immunosuppression, has been discontinued. While it was safe and well-tolerated in clinical trials, it did not meet efficacy and safety endpoints as measured by C-peptide, a biomarker for insulin production.
UP421 consists of islets derived from deceased donors that have been engineered to be hypoimmune, meaning they can avoid detection by the immune system without the need for immunosuppressants. Incredibly, the first person who received a partial dose of UP421, implanted in to forearm muscles, in a phase 1 clinical trial is making their own insulin, as demonstrated by increased C-peptide, without any side effects.
This is the first proof-of-concept evidence showing that this cell engineering approach can enable implanted islets to avoid immune destruction. The next step is applying this method to manufactured islets.
Breakthrough T1D is supporting research exploring similar cell engineering approaches to allow implanted islets to evade the immune system. The T1D Fund has also invested in Sana due to their distinctive hypoimmune manufactured islet replacement program, and Breakthrough T1D continues to work closely with them.
Tegoprubart is an immunotherapy that interferes with immune cell communication and dampens the immune response. This therapy is being tested in a Breakthrough T1D-funded phase 1/2 clinical trial as a novel anti-rejection immunosuppressant for people with severe hypoglycemia who have received deceased donor islets. Building on ongoing kidney transplant studies, this study will determine if tegoprubart can protect transplanted islets from rejection with fewer side effects compared to standard immunosuppressants, which is harsh on people and the implanted cells.
So far, of the first three participants, two have achieved insulin therapy independence. According to the study, tegoprubart is safer for both people and transplanted cells in comparison to standard immunosuppression, with milder side effects and greater islet survival. This study holds promise for preventing rejection of manufactured islets as well.
The T1D Fund has made several investments in Eledon to support this effort as it sees the potential to address the key unmet need of safe and effective immunosuppression for people who receive islet replacement therapies.
Cell Pouch™ is an implantable bio-hybrid organ that provides a specialized environment for transplanted islet cells by allowing them to access oxygen and nutrients provided by blood vessels, called vascularization.
The first cohort of a phase 1/2 clinical trial enrolled participants with severe hypoglycemia who received deceased donor islets within Cell Pouch in addition to standard immunosuppressants. Of the six enrollees, five remain insulin therapy independent from one year to more than five years. Cohort B is currently evaluating a higher-capacity Cell Pouch that can accommodate 50% more islet volume, and the trial will soon advance to Cohort C to further test safety and efficacy of the system.
Most excitingly, Sernova recently announced that following the conclusion of the ongoing clinical trial, they will initiate a new trial to test Cell Pouch implanted with manufactured islets—paving the way towards a scalable solution to T1D.
Breakthrough T1D has supported the development of Cell Pouch™ and continues to work with Sernova.
SR-02 is a manufactured islet cell therapy implanted onto the omentum, a fatty, protective layer around organs. This therapy is in a phase 1/2 clinical trial for people with severe hypoglycemia and requires immunosuppression. The trial is evaluating safety and insulin production as measured by C-peptide.
Seraxis is also working on another manufactured islet therapy (SR-03) that has been gene-edited so that anti-rejection immunosuppressants are not needed. They are hoping to initiate a new clinical trial for SR-03 in 2026.
The T1D Fund has invested in Seraxis to aid in the development of these therapies based on their distinctive and promising islet replacement approach.
CTX211 is another manufactured islet therapy that has been gene-edited to evade the immune system so that recipients do not have to take immunosuppressants. In an ongoing phase 1/2 clinical trial, these cells are implanted within a specialized device to help keep the cells alive in the body, and investigators are evaluating safety as well as insulin production measured by C-peptide. Results are expected this year.
Breakthrough T1D was a long-time supporter of ViaCyte, which initially developed the manufactured islets and partnered with CRISPR Therapeutics to genetically modify them. ViaCyte was acquired by Vertex in July 2022, and now CRISPR Therapeutics is the sole owner of this therapeutic platform.
- OPF-310 (Otsuka Pharmaceutical): encapsulated islets derived from pigs in phase 1/2 clinical trials
- ENC-201-CED (Encellin): donor-derived islets in a proprietary encapsulation device implanted subcutaneously in a phase 1 clinical trial
- Gastrin: a phase 1/2 trial testing if gastrin, a naturally occurring hormone involved in pancreatic development, is safe and helps retain or grow islets following donor-derived islet transplantation
Recruiting cell therapies clinical trials
| Therapy | Primary Outcome(s) | ID | Location | Phase |
| VX-880 (Zimislecel) | Safety, insulin independence, and absence of severe hypoglycemic events | NCT04786262 | US/Canada/UK/EU | 1/2/3 |
| VX-880 (Zimislecel) | Insulin independence in people with T1D and a kidney transplant | NCT06832410 | Canada | 2 |
| UP421 | Safety | NCT06239636 | Sweden | 1 |
| Tegoprubart + donor islets | Insulin independence | NCT06305286 | US (Chicago, IL) | 1/2 |
| Cell Pouch™ + donor islets | Safety | NCT03513939 | US (Chicago, IL) | 1/2 |
| SR-02 | Safety, C-peptide | NCT06651515 | US (Pennsylvania) | 1/2 |
| CTX211 | Safety, C-peptide | NCT05565248 | Canada | 1/2 |
| OPF-310 | HbA1c<7% and absence of severe hypoglycemic events | NCT06575426 | US (Chicago, IL) | 1/2 |
| ENC-201-CED ENCRT + donor islets | Safety | NCT06408311 | Canada | 1 |
| Gastrin + donor islets | Insulin independence, absence of severe hypoglycemic events, and HbA1c<6.5% | NCT03746769 | US (Duarte, California) | 1/2 |
Where do we go from here?
The emergence of cell therapies for T1D in clinical trials is incredibly exciting for the T1D community. Advancements using deceased donor-derived islets are paving regulatory pathways for manufactured islet therapies—which are curing people with T1D in clinical trials—to make their way towards the market.
“One of the greatest effects that manufactured islet cell therapies will have for the T1D community is being able to think about their type 1 diabetes less,” explained Nicholas Mamrak, Ph.D., a scientist at Breakthrough T1D. This may soon be a reality for a subset of people with T1D as we drive towards the approval of a first-generation manufactured islet replacement therapy, reducing the day-to-day burden of managing blood glucose and insulin dosing.
The ultimate goal is to make sure manufactured islet therapies are available to everyone with T1D—ideally without the need for immunosuppression. This is the objective of Breakthrough T1D’s Project ACT.
Project ACT
Scientific progress takes time, money, and effort. To accelerate islet replacement therapies faster than ever, Breakthrough T1D launched Project ACT (Accelerate Cell Therapies) to simultaneously advance research, development, regulatory policies, access, and adoption of manufactured islet therapies that do not require broad immunosuppression.
Before cell therapies can become available for everyone, they must first receive regulatory approval. A key part of Project ACT is streamlining and advancing regulatory pathways for the treatment of T1D. To help achieve this, Advocacy and Regulatory leadership teams at Breakthrough T1D are working on a new publication capturing how day-to-day lives have changed for people with T1D who have received cell therapies. By using personal experiences to share what benefits of cell therapies matter most to patients, we can help regulators understand the powerful impact that islet replacement therapies can have.
None of this would have been possible without the T1D community’s continued generosity and support, as we all work together to move the needle forward.
Have an impact by participating in clinical trials
Without clinical trials, we would never know if new therapies developed by scientists in the lab could make a difference in people’s lives. This is where the T1D community comes in—by volunteering to participate in clinical trials, you become uniquely positioned to help drive biomedical research forward. Moreover, by participating, you help not only yourself, but everyone with T1D. Find a clinical trial near you and see if you are eligible to participate. Connect with a Clinical Trial Education Volunteer in your area, who can answer any questions you may have.
They’re all over the news: Ozempic ®. Trulicity ®. Jardiance ®. Mounjaro ™. And more.
These drugs are all approved for glucose control in type 2 diabetes (T2D). Some of them also have additional indications reflecting their demonstrated benefits for cardiovascular disease, kidney health, and obesity.
None of these drugs are currently approved for people with type 1 diabetes (T1D). However, a recent consensus report addressed the growing interest in GLP-1 receptor agonists as an adjunctive therapy for T1D and their “potential to improve glycemic and metabolic outcomes without increasing the risk of severe hypoglycemia or diabetic ketoacidosis.”
Let’s examine GLP-1 therapies and SGLT inhibitors and consider how drugs like Ozempic may one day help people with type 1 diabetes.
GLP-1 therapies
GLP-1 (glucagon-like peptide-1) receptor agonists work in multiple ways to control blood glucose and obesity. They block the release of glucagon, stimulate insulin production, slow the rate at which your stomach empties, and increase the sensation of feeling full. They are usually injected, but oral versions are available.
In people with T2D, this class of drugs lowers blood sugar levels and, for most people, causes weight loss. GLP-1 therapies have also been shown to reduce the risk of long-term cardiovascular complications often experienced by people with T2D, such as heart attack and stroke.
GLP-1 drugs include Ozempic/Rybelsus/Wegovy (semaglutide), Trulicity (dulaglutide), Victoza (liraglutide), and Mounjaro (tirzepatide), among others.
When GLP-1 treatments hit the market in the early 2000s, Breakthrough T1D and others funded several clinical trials to test whether GLP-1 receptor agonists, in addition to insulin, improved outcomes for people with type 1 diabetes. While some of these studies showed that the addition of GLP-1 therapies to insulin led to improvements in HbA1c, total insulin dose, and weight, the benefits were relatively modest and accompanied by some side effects like hypoglycemia. As a result, these studies did not lead to GLP-1 receptor agonists being highly adopted for use by people with T1D.
However, these trials were done with older GLP-1 drugs. We are investigating whether the newest, most advanced GLP-1 therapies (like Ozempic) will improve the health of people living with type 1 diabetes. (See below!)
SGLT inhibitors
SGLT (sodium-glucose co-transporter) inhibitors are oral medications for people with T2D that lower blood sugar by preventing the kidneys from reabsorbing glucose, leading to the excretion of sugar through the urine.
In addition to improving blood sugar for people with and without T2D, these drugs also provide benefits such as weight loss, blood pressure reduction, and transformative benefits to the heart and kidneys.
SGLT drugs include Jardiance (empagliflozin), Farxiga (dapagliflozin), and Invokana (canagliflozin), among others. Despite demonstrating improved glucose control for people with type 1 diabetes, SGLTs have not been approved for people with T1D in the U.S. Increased risk of diabetic ketoacidosis (DKA) in this population limits the use of these therapies. A key Breakthrough T1D priority is to find ways to mitigate this risk so people with T1D can also take advantage of the SGLT cardiovascular and renal benefits.
Breakthrough T1D-funded research in GLP-1 and SGLT therapies
Breakthrough T1D has a long history with GLP-1 medications like Ozempic. In the 1980s, Breakthrough T1D-funded researcher Pauline Kay Lund, Ph.D., was the first to clone the hormone glucagon and discover two new hormones, one of which was GLP-1.
Today, there is real-world evidence of GLP-1 receptor agonists improving the lives of people with type 1 diabetes.
Real-world evidence
Observational studies using historical patient data from electronic health records (not randomized clinical trials) that can give an idea if a drug might be beneficial or not for a certain indication.
Evidence demonstrates that semaglutide (Ozempic) or tirzepatide (Mounjaro) have the potential to lower A1c, increase time-in-range, and reduce the amount of daily insulin needed in people with T1D. More research is needed in this area. That’s where Breakthrough T1D comes in!
Breakthrough T1D-funded research on GLP-1 and SGLT therapies is investigating the benefits of these drugs for people with T1D, including reducing the risk of common complications of type 1 diabetes like cardiovascular disease and kidney disease.
Snapshot of active clinical trials in GLP-1 and SGLT therapies
Here are a few examples of Breakthrough T1D-funded clinical trials in GLP-1 and SGLT therapies that are currently underway:
| Clinical Trial Name | Study Details |
|---|---|
| REMODEL T1D | Determine whether semaglutide (Ozempic) protects the kidneys in those living with T1D. |
| Triple Therapy in T1DM | Assess whether the addition of dapagliflozin (Farxiga) to semaglutide (Ozempic) and insulin improves glycemic control in those living with T1D. |
| SUGARNSALT | Determine the effectiveness and safety of sotagliflozin (Inpefa) in slowing kidney function decline in those living with T1D and moderate to severe diabetic kidney disease. |
| Dapagliflozin + Pioglitazone in T1D | Examine how adding dapagliflozin (Farxiga) and pioglitazone (Actos) to insulin therapy affects glucose control and ketone concentration in people living with T1D. |
Our commitment to improving lives
Breakthrough T1D strives to improve health outcomes in people living with type 1 diabetes. Insulin therapy alone is often not enough for people with T1D to achieve glucose and metabolic control. The use of adjunctive drugs that complement insulin therapy can help. Since the FDA has already approved these medications for treating other conditions, positive results from these clinical trials could speed up the adaptation of these therapies for people living with T1D.
Learn more about clinical trials and how they are advancing breakthroughs for the T1D community.
After months of unexplainable symptoms, Katie Howell was diagnosed with type 1 diabetes (T1D) last year at age 25. Read on to learn more about how she confronted her new reality and became the first participant in New York City to enroll in the DIAGNODE-3 clinical trial.
The unexpected diagnosis

Katie Howell, a Mississippian-turned-New Yorker, has a wide array of hobbies: crafting pottery at her local ceramics studio, enjoying old movies in the theater, cozying up with a book in Prospect Park, and taking in the sun at Rockaway Beach. Katie moved to Brooklyn after completing a Master of Public Administration degree at the University of Tennessee, Chattanooga, and she’s thoroughly enjoying getting to know her new neighborhood.
Suddenly, things took a turn. Last year, Katie started experiencing symptoms of something unknown—for three months, she had no idea what was going on with her body. Then, in a moment of complete shock, 25-year-old Katie was diagnosed with type 1 diabetes (T1D). “Without a family history of T1D and without any health issues of my own, being diagnosed with a sudden, serious chronic illness could not have been more unexpected,” Katie explained.
At the time of her diagnosis, Katie was confronted with a flood of emotions. There was not a single person in her life that had T1D or could understand her experience. “…one of the most challenging parts of being diagnosed with a chronic illness is accepting it […]. This comes with a lot of hopeless feelings, and it is tough to surrender control to an incurable, pervasive, and expensive health condition,” Katie lamented.
Feeling alone and lost, she had no choice but to accept that she was dealing with a major life change—whatever that meant for the future.
Influenced
It wasn’t long before Katie took to social media to learn more about her diagnosis and connect with the T1D community. On Instagram, she stumbled upon Lauren Bongiorno, a T1D influencer. Ms. Bongiorno posted a video promoting DIAGNODE-3, a phase 3 clinical trial for the disease-modifying therapy Diamyd® for early-stage T1D.

Katie found that DIAGNODE-3 was enrolling at The Pediatric Diabetes Center at Hassenfeld Children’s Hospital at NYU Langone. After reaching out to the team at NYU, she realized that the stars had aligned: they had just opened enrollment, and after completing the necessary screening, Katie would be Participant Number One.
“It gets easier every visit!”

Katie received her first study injection in January and will get her third and final injection this month, followed by routine check-ins. This includes blood work, physical exams, and mixed-meal tolerance tests to measure her body’s ability to produce insulin.
As per the study protocol, Katie doesn’t know if she’s receiving the placebo or the study drug, Diamyd®. Still, she likes being in the study: she’s made meaningful connections with the trial team, has learned a lot about T1D, and feels that she’s making a difference—not just for herself, but also for biomedical research.
Katie’s biggest hurdles? Bloodwork and hospitals. “One challenge of this study is the routine blood work and being treated at the hospital. However, it does feel like exposure therapy. It gets easier every visit!” she exclaimed.
Finding her community
Since her diagnosis, Katie has made lasting relationships with others in the T1D community. She connected with the Greater New York Metro Chapter of Breakthrough T1D after volunteering at a Walk in NYC last fall. They introduced her to a group chat with other newly diagnosed young women, where they can ask questions, network, and learn from each other as they navigate their new realities.

“My advice to someone considering [the DIAGNODE-3] trial would be that, though the study is a commitment and an undertaking, they are taking part in discovering medical breakthroughs and T1D treatment research.”
Katie Howell
Participate in clinical trials
Clinical trials are key to bringing medical advancements from the lab to the clinic. This wouldn’t be possible without brave people with T1D, like Katie, who volunteer to participate. These studies offer the potential for life-changing treatment and move the ball forward for the T1D community.
Use our Clinical Trials Matching Tool to find a trial near you. Connect with a Clinical Trial Education Volunteer in your area to learn more about trial participation and answer any questions you may have.

When Tzield was approved by the United States Food and Drug Administration (FDA), the type 1 diabetes (T1D) community had real cause to celebrate: The first disease-modifying therapy for T1D had cleared one of the last major hurdles to becoming available.
Disease-modifying therapies
Also "DMTs" for short, these therapies prevent, slow, halt, or reverse T1D progression.
But once Tzield was on the market and covered by health insurance companies and other payers, a new hurdle emerged: a majority of healthcare providers across the country were unaware of the drug, let alone how to administer it.
The clinical guideline for Tzield infusion did not become available until a year and a half after the FDA approved the drug. To date, 500 people in the U.S. with early stage T1D have received Tzield. Compare that to the annual incidence rate of T1D in the U.S. according to the T1D Index:
According to a 2023 study in the journal Diabetes Technology & Therapeutics, Tzield isn’t the only advanced T1D therapy with a surprisingly low adoption rate.
The FDA approved the first artificial pancreas (AP) system in 2016. Less than a decade later, there are now eight such approved systems on the market. These systems—also called automated insulin delivery (AID) systems—lead to better T1D management and health outcomes—yet only 16 percent of people with T1D in the United States use them.
Similarly, the FDA approved Lantidra, the first donor-derived cell therapy for T1D, in 2023. To date, one person has received it.
Increasing adoption to improve health
Closing the gap between access to and adoption of T1D therapies is a mission priority for Breakthrough T1D.
“It’s similar to the question: ‘If a tree falls in the forest and no one is there, does it make a sound?’” said Anastasia Albanese-O’Neill, Ph.D., APRN, CDCES, Associate Vice President of Breakthrough T1D’s Community Screening and Clinical Trial Education programs. “In this case, if you have a cutting-edge new therapy but most healthcare providers don’t know about it, don’t prescribe it, and don’t know how to administer it, does it have an impact?”
The organization recently announced the establishment of a Medical Affairs unit. The team will address the numerous challenges contributing to the slow adoption of groundbreaking T1D therapies, delaying their life-changing potential for many people living with the disease.
Challenges we are addressing:
HCPs have much greater knowledge of type 2 diabetes—or T2D—which is more prevalent.
HCPs need comprehensive guidelines to support new, approved treatment options.
There are too few clinical environments with the equipment and expertise to administer advanced T1D therapies and treatments, such as new T1D devices, therapies that require infusions like Tzield, and treatments that require implantation, such as cell therapies.
There are too few endocrinologists and certified diabetes care and education specialists with knowledge and competency in advanced T1D therapies.

With the establishment of our Medical Affairs team, we are reaffirming our organization’s commitment to creating a world where every individual with type 1 diabetes has access to life-changing therapies. By addressing systemic barriers and fostering clinical readiness, Breakthrough T1D will be pivotal in driving the timely adoption of emerging therapies and transforming care.”
As part of this organizational change, the Community Screening and Clinical Trial Education team, led by Albanese-O’Neill, will be integrated into Medical Affairs.
The team will focus on developing education materials for healthcare professionals in the U.S. and around the world; empowering people with T1D to participate in shared decision-making with their healthcare teams about emerging T1D therapies; helping to establish and socialize clinical care guidelines tailored to regional needs; and expanding clinical trial participation through community activation and HCP education.
“We have been doing a great deal of work to expand our HCP education, T1D community screening, and clinical trial education programs for more than three years now,” said Albanese-O’Neill, who has been with Breakthrough T1D as a fulltime staff member since 2022. “Given what we are seeing with adoption rates and with Dr. Danne joining us, we are now putting all of this work together in one department with a more strategic approach.”

Empowering clinicians with education
The team recently launched comprehensive, expertly redesigned HCP education and training resources.
These resources—which are accredited, free-of-charge, and live or on-demand—offer a significant focus on early detection for the earliest stages of T1D, monitoring guidance for positive test results, clinical trial opportunities, and the latest on cutting edge T1D therapy research and development, including disease-modifying therapies and islet cell therapies.
While designed specifically for healthcare professionals who can earn 4.5 credit hours of continuing medical education, the resources are available to the public. The on-demand feature means busy healthcare professionals with schedules that include all kinds of shifts imaginable can access this turn-key resource on their own time.
For a deeper dive, Breakthrough T1D’s resources will also offer live sessions, allowing time to interact with and learn from leading experts in the T1D field, including Albanese-O’Neill and Danne, in addition to those affiliated with different clinical facilities and institutions across the nation.

Our goal is to make this education as accessible as possible.”
Detecting T1D before symptoms present
A simple blood test can detect T1D in the earlier stages—before obvious symptoms develop. The biggest challenge is educating clinicians and the general population about it.
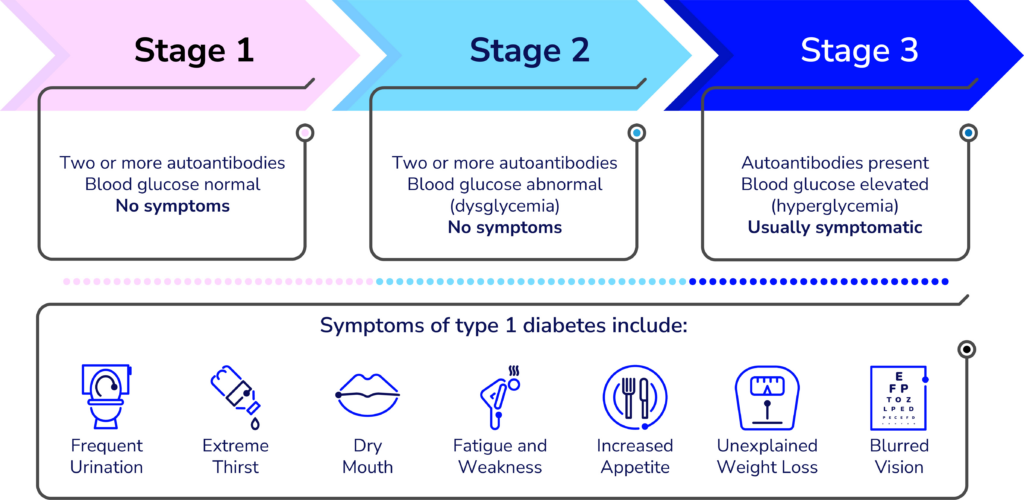
“Endocrinologists, Pediatricians, and some other specialty physicians learn about T1D screening and monitoring during their residencies, but it’s not a part of the general curriculum of the first four years of medical school,” said Lally, who built the learning management system for the new resources and is organizing the virtual offerings. “We’re working to advance that knowledge to yes, doctors, but also other clinicians whose patients could benefit.”
Many clinicians hesitate to order unfamiliar tests—especially if they are unsure what to do with the results. Most people who see any kind of healthcare provider could benefit from screening for T1D—according to a paper published in the journal US Endocrinology, roughly 85 percent of people diagnosed with type 1 do not have a blood relative with the autoimmune disease.
“Clinicians need to learn about the stages of type 1 and the specific autoantibody tests that identify type 1 versus type 2 and identifying type 1 in individuals at risk before they need insulin,” said Colleen Buggs-Saxton, M.D. Ph.D.
Buggs-Saxton, a Pediatric Endocrinologist at Wayne Pediatrics in Michigan, is the clinical leader of a Breakthrough T1D Early Detection pilot clinic. Using the new resources, she and Albanese-O’Neill are going to lead a grand rounds about T1D early detection at her institution, which is affiliated with the Wayne State University School of Medicine and healthcare system.

Clinicians should consider autoantibody testing for adults who have been diagnosed with type 2 but don’t have typical clinical features and require insulin to manage their blood sugars.”
“This is a novel way these resources can be used—as the basis of a locally and or virtually-provided grand rounds,” said Albanese-O’Neill.
While much of the emphasis of T1D early detection programs has been on children and teens, its applications are much broader—anyone can develop T1D at any age and unfortunately, misdiagnoses happen. According to an article published in the journal The Lancet, Regional Health: Europe, it is estimated that nearly 40 percent of adults older than age 30 with T1D may have been misdiagnosed with T2D.
“Most clinicians are very comfortable ordering an HbA1c test to classify people with type 2 diabetes, but they do not know what tests to order to classify people with type 1,” added Buggs-Saxton.
Grand rounds
Grand rounds are educational meetings and presentations for clinical teams at a given institution or healthcare facility to provide a summary of updates to the standards of care.

What to do with positive test results
The screening test is just the first part of T1D early detection. Clinicians also need to know what to do with positive results once they come in. Breakthrough T1D’s HCP resources offer extensive education on the topic.
Less than one year ago, Breakthrough T1D and other leading diabetes organizations developed monitoring guidance to help clinicians support people who test positive for stage 1 or stage 2 T1D. The guidelines have been endorsed by leading medical journals and organizations around the world.
”This monitoring guidance can help any clinician feel confident in providing adequate care in the early stages of type 1 and know when to refer to a specialist,” said Albanese-O’Neill.

I think of research, advocacy, and medical affairs as three legs of a stool—in terms of clinical adoption, each helps answer a different question. Research: Does it work? Advocacy: Will the regulators approve it and will insurance companies cover it? Medical Affairs: Do clinical teams have the competency and readiness to prescribe the treatment and educate and support people with T1D?”
It’s also helpful for the people who test positive for early stage T1D. Using the monitoring guidance, people can work with their healthcare team to monitor blood glucose levels to identify when insulin therapy may be needed; consider participating in clinical trials of disease-modifying therapies in development; and consider when and whether Tzield might be appropriate.
“There is currently one FDA-approved disease-modifying therapy for early-stage type 1 diabetes and additional therapies are being studied in clinical trials,” said Lally. “Identifying type 1 early gives the individual and their family time to learn more about type 1 and their options before reaching stage 3 T1D, which requires daily insulin therapy.”

Clinical trials: Increasing patient referrals
Clinical trials are a vital step for any treatment, drug, or device to make it into the hands of people with T1D. Currently, more than 300 clinical trials focused on T1D are actively recruiting participants.
Moreover, clinical trials can offer people the chance of receiving a cutting-edge treatment they may not otherwise be able to access.
Through its HCP resources and existing clinical trial resources, Breakthrough T1D is stressing the significance of investigational T1D therapies—while also clarifying common misconceptions about clinical trials.
“The clinical trial education portion of the program explains current trial opportunities and the critical need to increase diversity in diabetes research,” said Lally.
Despite the importance of clinical trials, many are delayed due to slow enrollment, adding cost and prolonging the results. A 2020 Tufts University study found that nearly 90 percent of clinicians surveyed felt comfortable talking about clinical trials.
“Unfortunately, the survey also revealed that annually, fewer than one percent of patients are referred to clinical trials,” said Lally.
So, why aren’t more clinicians referring their patients to clinical trials? Time and resources.

The most challenging part is helping patients understand what a clinical trial is, what it involves, and how previous scientific advances were only possible because of clinical trials. Healthcare providers often don’t have enough time in their busy clinics to discuss this with patients and families.”
Jacobsen, who is with University of Florida Health (UF Health), is one of the faculty members for Breakthrough T1D’s new HCP resources—specifically, the offering related to currently recruiting clinical trials for T1D disease-modifying therapies.
Jacobsen stresses that families and individuals with T1D also need specific education on the potential of receiving a placebo during a clinical trial—and why it’s an impactful part of any clinical trial.
Clinicians also may not know how to quickly get and stay up to date on current trial opportunities and how to get individuals who test positive for T1D autoantibodies involved.
“We provide a streamlined presentation about how to talk to families of people with T1D or people at risk for T1D about clinical trials,” explains Jacobsen, “We can direct families to one of several websites for more detailed information, like Breakthrough T1D’s Clinical Trial Finder.”

Cell therapies: Cures within reach
Cell therapies are one of the most promising approaches to curing T1D and one of the cornerstones of Breakthrough T1D’s cures research portfolio.
Advances in cell therapies have ramped up in recent years: participants in clinical trials of these therapies have been able to stop taking insulin. To speed this progress even more, Breakthrough T1D recently launched Project ACT (Accelerate Cell Therapies).
Cell Therapies
Also called islet cell therapies, these therapies replace destroyed beta cells so that people with T1D can again produce their own insulin.
The organization has long invested in cell therapy research and has a track record of success in making life-changing T1D therapies a reality—the prime examples being artificial pancreas systems (AP systems) and Tzield.
The work of Breakthrough T1D’s Research, Advocacy, and Medical Affairs teams—in partnership with the organization’s venture philanthropy fund, the T1D Fund—will be integral to Project ACT’s success.
Like AP systems, Tzield, and all other FDA-approved drugs, treatments, and medical devices on the market today, islet cell therapies will only become available after meeting all the required benchmarks—including clinical trials.
Clinicians who are in-the-know about clinical trials and how to help their patients enroll are but one of the numerous ways Breakthrough T1D’s Project ACT will make islet cell therapies a reality, faster.
“Clinicians are generally the most trusted source for this information, but most are not making those referrals, so the gap never closes,” said Lally. “We aim to change that.”
“We want every member of the diverse T1D community to be aware of clinical trials, how to participate, and where to find information,” added Albanese-O’Neill. “The next generation of breakthroughs depends on it.”
Editor’s note: This story co-written by Ginger Vieira, special contributor to Breakthrough T1D.
Marjorie Lazarre (R) with her daughter, Ava (L)
A life-changing event at age 14 determined Marjorie Lazarre’s career.
“My sister was diagnosed with type 1 diabetes (T1D) after being rushed to the hospital,” she recalled. “I decided to go into pharmacy as a way to better understand medication and educate myself to support her, as well as other patients.”
Marjorie received her doctoral degree in pharmacy from Howard University in Washington, D.C., and completed a pharmacy practice residency at Yale New Haven Hospital. She currently serves as the Director of Pharmaceutical Procurement and Business Practices at Yale New Haven Hospital.
Another life-changing event
Several years ago, Marjorie and her husband received devastating news: their 7-year-old daughter, Ava, had type 1 diabetes. “Again, my life changed,” she said. She started looking for ways to help improve her daughter’s life beyond the medical field. “I wanted to dedicate my effort not only to understanding medication but supporting the advocacy and philanthropy efforts of Breakthrough T1D.”
Marjorie joined her Breakthrough T1D Community Board, and her family became a top fundraising team for their local Walk. They also helped with support groups and community outreach events. Still, they wanted to do more.
Clinical trials: A path to cures
Marjorie saw firsthand the “tremendous shift from cumbersome needles and finger sticks” to continuous glucose monitors (CGMs) and insulin pumps. She knows none of these advancements would have been possible without clinical trials. So, her family started taking part in a variety of type 1 diabetes clinical trials, ranging from simple surveys to mental health analysis to blood tests.
She then became a Breakthrough T1D Clinical Trial Education Volunteer, helping families find clinical trials that are a good fit and increasing participation to fuel research for T1D. “Clinical trials are the only way to get to a cure,” she said. “The more folks that participate, the more data we have to improve and optimize treatment options.”
Marjorie is hopeful that her next life-changing event will be a cure for T1D. “I see continued improvement to the quality of life through devices and medicines,” she said. “To find a cure for type one diabetes would change so many lives.”