
100 Years, 100 Breakthrough T1D Scientists: Gian Franco Bottazzo, M.D.
In honor of the 100th anniversary of insulin, we are launching “100 Years, 100 Breakthrough T1D Scientists.” The first up: Gian Franco Bottazzo, M.D.
CDC Study: Children Who Have Recovered from COVID-19 May Be at Increased Risk of Diabetes
CDC researchers report an apparent association between pediatric COVID-19 and diabetes diagnoses.

Celebrating the Best of 2021
In 2021, Breakthrough T1D has seen tremendous progress in accelerating cures, improving lives, and advocating for people with type 1 diabetes and their loved ones.

Targeting a New Hormone Helps to Prevent Type 1 Diabetes
A publication authored by Harvard University scientists has found that targeting a new hormone, FABP4, can enhance beta cell function and prevent T1D.
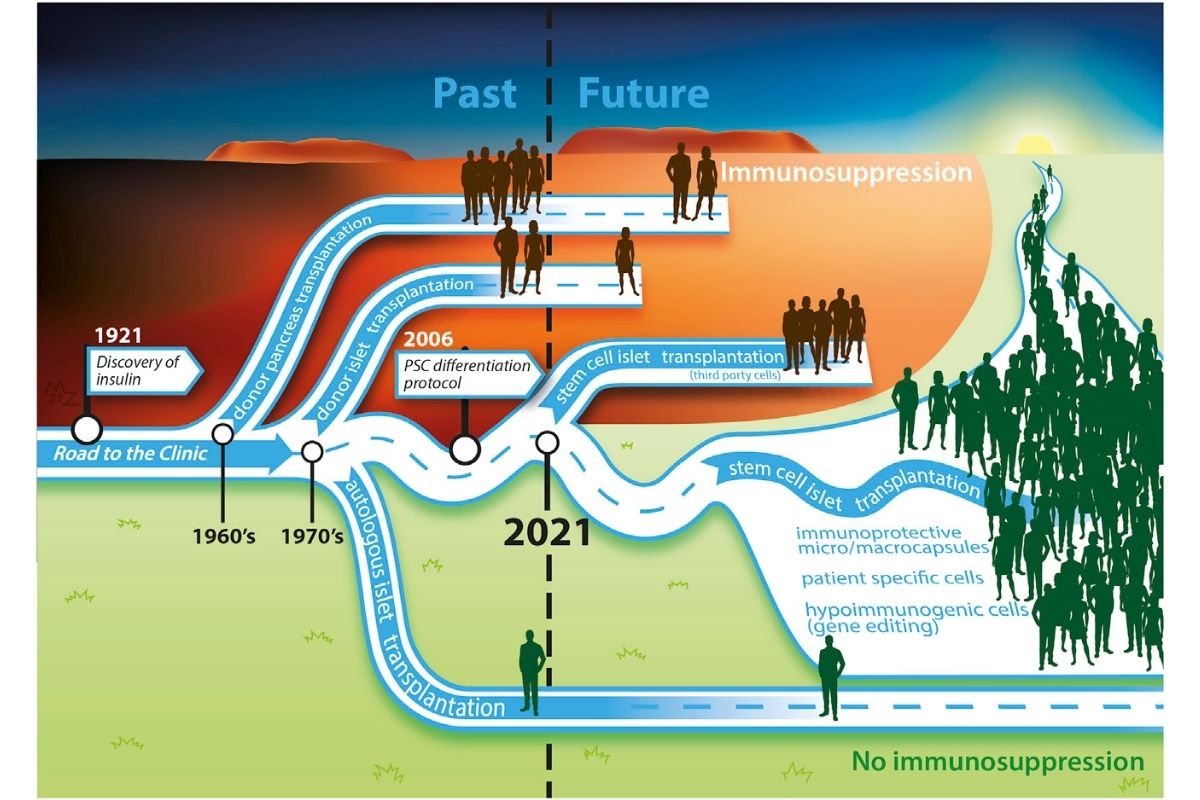
Stem Cell Replacement Therapy: Its Time has Begun
ViaCyte has results on its second stem cell-based technology, providing evidence of stem cells secreting insulin in response to meal consumption.

Progress Toward Cures: Thoughts at Year’s End from Breakthrough T1D’s CEO
Breakthrough T1D CEO Aaron Kowalski, Ph.D., weighs in on the exciting news surrounding stem cell-derived beta cell replacement therapies, now in human clinical trials.

Find Your Something
A resident physician and T1D dad—who also lives with an autoimmune disease—shares his call to action to support Breakthrough T1D and research to cure T1D and improve lives.
First-in-Human Gene-Edited Stem Cell Trial? Check.
ViaCyte, a beta cell replacement company long supported by Breakthrough T1D, and CRISPR Therapeutics have a new first: Gene-editing for type 1 diabetes.
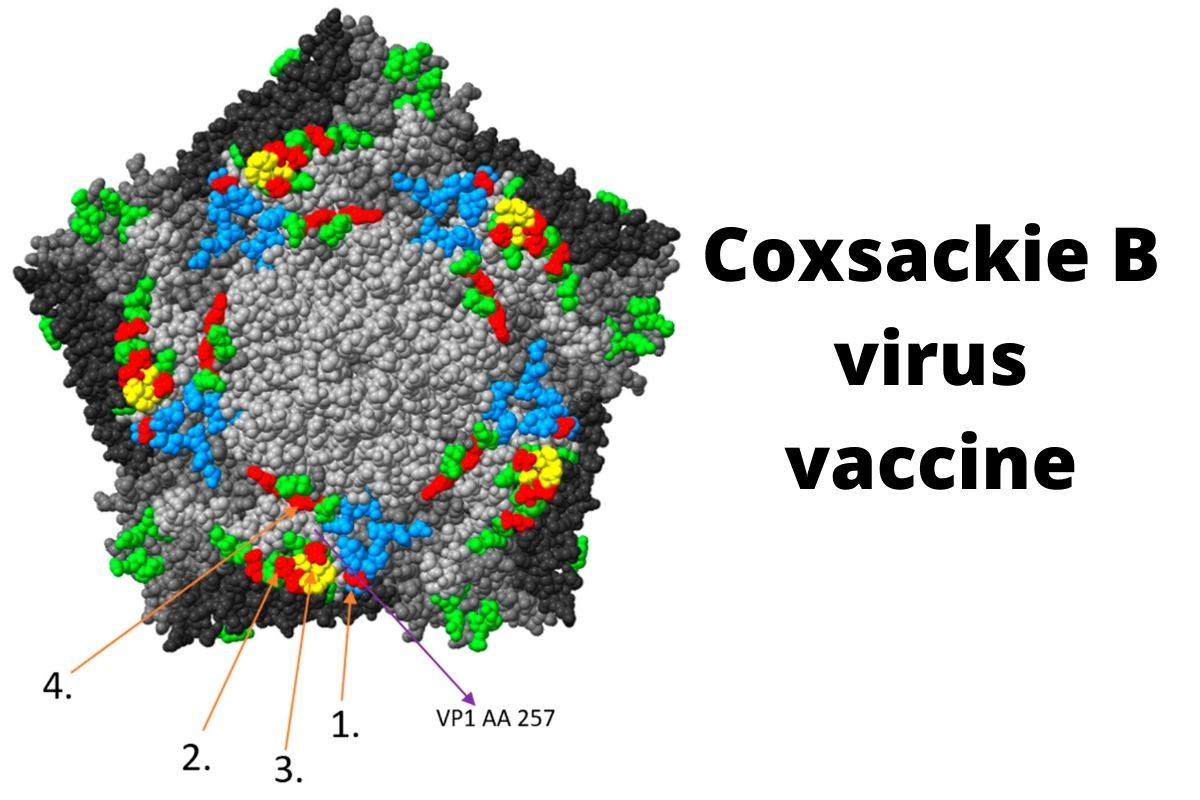
First-in-Human T1D Vaccine Trial Reports Positive Results
A first-in-human study of the coxsackie B vaccine—made by Breakthrough T1D-funded researchers Drs. Hyoty and Knip—reports positive results.

Artificial pancreas systems: Sleeping without fear
Research shows Artificial Pancreas Systems can help improve diabetes-related outcomes and lighten the burden of T1D. Even during sleep.