The U.S. Food and Drug Administration (FDA) cleared the iLet® Insulin-Only Bionic Pancreas System, which is designed to autonomously determine and deliver insulin doses to control blood-sugar levels, for people 6 years of age and older with type 1 diabetes (T1D). It includes an algorithm and an integrated infusion pump, which communicates directly with a compatible FDA-cleared integrated continuous glucose monitor (iCGM), enabling it to be an artificial pancreas, or automated insulin dosing (AID), system.
What’s new about this system? Ease-of-use. The iLet system is designed to have users enter only their weight for the iLet to initialize therapy. Immediately thereafter, the iLet begins controlling blood-sugar levels automatically, without requiring the user to count carbohydrates, set insulin delivery rates, or deliver additional insulin for meals or corrections. (Users do have to say whether the amount of carbs in a meal is small, medium, or large, but the algorithm learns over time in response to their individual insulin needs.)
The submission was based on a multi-center randomized insulin-only iLet Bionic Pancreas pivotal trial, which tested the insulin-only configuration in 440 adults and children 6 years and older with T1D. The trial met all key endpoints, demonstrating improved outcomes over standard of care for people living with T1D:
- Average HbA1c fell from 7.9% to 7.3% at 13 weeks
- An average of 2.6 hours more time-in-range (70-180 mg/dL) per day, improving from 51% to 65% at 13 weeks
- No increased risk of hypoglycemia
There are now multiple artificial pancreas systems on the market: The Medtronic 670G (2016), Tandem Control-IQ™ (2019), Medtronic 770G (2020), Insulet Omnipod 5 (2022), Medtronic 780G (2023), and, now, the iLet® Insulin-Only Bionic Pancreas System. (Tidepool Loop, an app that contains an algorithm that automates insulin dosing, has also been approved, but it has not yet announced its insulin pump manufacturer.)
Breakthrough T1D Impact
Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with T1D have better, more innovative ways to manage their type 1 diabetes until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation. To date, Breakthrough T1D has funded more than $140 million in artificial pancreas research.
Through these grants, Breakthrough T1D supported the development of the algorithm and preclinical and early clinical research—in partnership with the Helmsley Charitable Trust—through grants to:
- Firas El-Khatib, Ph.D., who received a Breakthrough T1D postdoctoral fellowship from 2006-2007 and is now co-founder and VP, Research & Innovation, at Beta Bionics
- Ed Damiano, Ph.D., co-founder and executive chair of Beta Bionics, from 2009-2011
- Steven J. Russell, M.D., Ph.D., who received a grant from 2013-2016 and is the principal investigator on all of the iLet clinical trials
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
The Food and Drug Administration (FDA) recently approved the Medtronic MiniMed™ 780G artificial pancreas system for use in individuals 7 years and over. It provides automatic adjustments and corrections to blood-sugar levels every 5-minutes, and also correction doses, as part of its meal detection technology.
This is an evolution of the 670G, the world’s first artificial pancreas system, which was approved in 2016, and an updated version, 770G, which was authorized in the United States in 2020. (The 780G was approved in Europe in 2020.)
In the pivotal clinical trial:
- Time-in-range (blood sugar between 70-180 mg/dL) was 75 percent, helping those with T1D maintain more consistent and healthier glucose levels
- The overall time-below-range was 1.8 percent, and overnight it was 1.5 percent
- Overnight time-in-range was 82 percent
The 780G is approved for use with the Medtronic Guardian 4 continuous glucose monitor (CGM). Unlike its predecessor, the Guardian 3, the Guardian 4 does not require fingerstick calibrations.
The 780G is also approved for use with the Medtronic Extended Infusion Set (EIS), which can be used for seven consecutive days (most infusion sets can be used for 3 days).
Per Medtronic, individuals will be able to pre-order the device on May 15 with an expected delivery in June.
Breakthrough T1D Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with projections for next generation versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project almost 20 years ago to ensure that people with T1D would have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The Breakthrough T1D Artificial Pancreas Project and the Breakthrough T1D Artificial Pancreas Consortium have dramatically accelerated progress by bringing together academic researchers, government agencies, industry partners, and the Helmsley Charitable Trust to pursue artificial pancreas technology.
Breakthrough T1D has funded over $140 million to date in artificial pancreas research.
- Five algorithm teams of the Breakthrough T1D Artificial Pancreas Consortium and Tidepool developed algorithms that made the Medtronic MiniMed 670G, 770G, and 780G, Tandem Control-IQ™, CamAPS® FX, Insulet Omnipod 5, and Tidepool Loop
- Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system, the first artificial pancreas system, which was approved in 2016.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management until cures are found.
Cynthia Rice, Breakthrough T1D’s Chief Mission Strategy Officer, will leave behind quite a legacy once she steps down from her role at Breakthrough T1D at the end of March 2023.
“In her time with Breakthrough T1D, she has led with strategic purpose and passion,” read Breakthrough T1D CEO Aaron Kowalski’s December 2022 memo announcing Cynthia’s decision to leave. “She has been an incredibly valuable partner to me, as well as staff and volunteers throughout the organization.”
During her tenure at Breakthrough T1D, she has helped bring the artificial pancreas project to life, has driven efforts to renew the Special Diabetes Program, and was a key player in Breakthrough T1D’s response to and handling of COVID-19—all with the partnership of our strategic staff and vast network of volunteers, who are the bedrock of our advocacy efforts.
“It’s possible—while challenging—to impact the research and development (R&D) ecosystem to improve options and outcomes for people living with chronic diseases like type 1 diabetes,” says Cynthia. “Defining goals, tapping into strengths, building capacities, and remaining determined in the face of obstacles are critical.”
And for nearly two decades, she has done just that at Breakthrough T1D.
“Leverage—enlisting others to our cause—is critical to our success and core to our organizational DNA, whether it’s engaging friends and families, company R&D heads, government officials, or foundation leaders,” Cynthia says.
The Artificial Pancreas Endeavor

From Left: Breakthrough T1D International Board of Directors member Claudia Graham, Ph.D., M.P.H.; Breakthrough T1D Chief Mission Strategy Officer Cynthia Rice; and Senator Susan Collins (R-ME). Left-click on image to slightly enlarge.
Cynthia came to Breakthrough T1D in September 2005. Real-time continuous glucose monitors (CGMs) were in the early stages of development, with one approved just months prior.
Aaron Kowalski, Ph.D., who had come to Breakthrough T1D a year before and is now the CEO, and Jeffrey Brewer, a member of Breakthrough T1D’s International Board of Directors at the time, had just spent six months interviewing academic scientists, corporate executives, and other like-minded players to figure out whether Breakthrough T1D wanted to take on the development of an artificial pancreas. There were many barriers, and companies were very wary of getting involved.
In the interviews, it became clear that despite the hesitation among companies, there was significant potential benefit for the T1D community in pursuing artificial pancreas technologies. The leadership—needed to foster a therapy roadmap, research funding, regulatory pathway, and health care access—just wasn’t there.
Breakthrough T1D changed that. We made it a priority, bringing not only our research funding, but also our powerful advocacy forces, to speed the development of these devices.
“The goal of multiple artificial pancreas systems, with ongoing innovation, drove our strategy,” says Cynthia, “and we took actions early on with that goal in mind, utilizing our strengths, building new capacities and relationships, and battling doggedly to overcome obstacles.”
Overcoming the Obstacles
Among the first obstacles was that the benefits of continuous glucose monitoring in the management of T1D had only been shown in small studies. In 2008, a Breakthrough T1D multi-site randomized control clinical trial showed that people with T1D who used the devices experienced significant improvement in blood-sugar control. This was instrumental to CGM coverage and laying the groundwork for the artificial pancreas system to come to fruition and be covered by the healthcare system.
Another obstacle was linking together the two main components of a closed-loop artificial pancreas system—the glucose sensor and an insulin pump. Breakthrough T1D established the Artificial Pancreas Consortium, which connected researchers from several different laboratories to develop the computer algorithms so that the machines could “talk” to each other and then be commercialized, as necessary.
A third obstacle—perhaps the most challenging of them all—required engaging government, regulatory, and health care groups.
Breakthrough T1D worked with researchers, insurance companies, the National Institutes of Health (NIH), the U.S. Food and Drug Administration (FDA), Medicare, and Congress on regulatory and coverage issues. When the first artificial pancreas system came on the market in 2016, the T1D community was more than ready for the life-changing T1D management it offered.
“Seeing the artificial pancreas go from concept to reality, which is helping so many people keep their blood-sugar management in control, is what makes Breakthrough T1D and all of the advocacy volunteers—who sent an email, made a call, signed an action alert, or met with their Member of Congress—very proud of this historic achievement and the impact that these will have on the individual lives of those with type 1 diabetes,” Cynthia adds.
The Special Diabetes Program (SDP)

Cynthia Rice (far right, fifth row from the back), Breakthrough T1D volunteers, and Delegates at Children’s Congress 2013 with then-Vice President of the United States Joseph R. Biden (center). Left-click on image to slightly enlarge.
In 1997, with the bipartisan leadership of White House Chief of Staff Erskine Bowles and Speaker Newt Gingrich, Congress created the Special Diabetes Program (SDP), which annually allocates $150 million for T1D research at the NIH. Breakthrough T1D is the chief advocate of the SDP.
The SDP has been instrumental to some of the greatest advancements in the history of T1D—including research that led to artificial pancreas systems and the recent FDA approval of the first-ever drug that can delay onset of T1D, Tzield™ (teplizumab-mzwv).
Since its inception, the SDP has invested $3.4 billion into T1D research. The program’s success and continued renewal is the result in part of hundreds of Breakthrough T1D advocates meeting with their Members of Congress each year to discuss the importance of the SDP.
“Sustaining bipartisan support to renew again and again in challenging times in Washington is thanks to the amazing volunteer-staff partnership in advocacy,” says Cynthia. “This is now paying enormous dividends, not only in the artificial pancreas systems, but in cures therapies, including disease-modifying and cell therapy treatments.”
Breakthrough T1D’s Unique Strengths
“Breakthrough T1D has two strengths that are rare,” says Cynthia. “The first is scientific expertise, convening the best and brightest across fields and generating ideas to solve the biggest problems. The second is community passion, to influence R&D priorities, regulatory pathways, and health care access and enlist government leaders to take action for our cause.”
She adds: “Breakthrough T1D has harnessed these strengths and organized the community, leading to our higher-level power: Influence.”
“It’s not only possible but realizable for a small band of determined people, starting with our founding moms, to tackle and overcome big obstacles,” says Cynthia. “As long as we organize ourselves well, deploy smart strategies, and develop an advocacy message that people can get around, Breakthrough T1D will continue to have the impact that has historically been the pillar of our advocacy work.”
“More broadly, strong patient advocacy strengthens our health system and our society and helps align incentives in research, development, and health policy to benefit the people affected by the disease,” says Cynthia. “All of us as leaders should be thinking about what else we can do to help strong, independent patient communities come together and thrive as advocates, which I hope to do when I return to the health sector in 2024 after a sabbatical.”
A Legacy of Women Leaders
Breakthrough T1D was founded by women, has mostly women staff and volunteers, and counts numerous successful and influential women among its current and past leaders and supporters.
Women who, like Cynthia Rice, share Breakthrough T1D’s vision for a world without T1D and who will stop at nothing to turn that vision into reality.
“From the majority staff and volunteer base, to our women founders, to our international chair Mary Tyler Moore, to our advocates, fundraisers, and scientists,” says Cynthia, “Breakthrough T1D, as an organization, shows the power women can have to impact their world.”

Members of Breakthrough T1D’s Grassroots Leadership Team (GLT) along with members of Breakthrough T1D’s Advocacy Team, including Cynthia Rice (ninth person in from the right) at Breakthrough T1D Government Day 2023. Left-click on image to slightly enlarge.
The U.S. Food and Drug Administration (FDA) authorized the Tidepool Loop, an automated insulin dosing app intended for the management of type 1 diabetes (T1D). The Loop is, essentially, an algorithm that can, eventually, be used to work with commercially available insulin pumps and continuous glucose monitors (CGMs). The goal of the interoperable design: Provide flexibility for users and their healthcare teams to choose the compatible components that work best for them in managing their care.
It will be available as an app on iOS, to enable insulin delivery from a compatible Apple Watch. Tidepool has not yet announced its initial launch device partners, but the company has a development partnership with Dexcom and additional yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform.
Breakthrough T1D Impact
Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with type 1 diabetes have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation. To date, Breakthrough T1D has funded more than $140 million in artificial pancreas research.
Through these grants, Breakthrough T1D supported the development of the algorithm and other open source programs—in partnership with the Helmsley Charitable Trust—through grants to Tidepool.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
Here is Aaron J. Kowalski, Ph.D., announcing the approval, highlighting Breakthrough T1D’s role in the research and regulatory process, and reiterating that people with T1D should connect with their physician about the treatments best for them:
To learn more, visit the Tidepool Loop website.
The Food and Drug Administration (FDA) authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system, for children ages 2+. It includes an algorithm placed in a waterproof, closed loop insulin pump and communicates directly with a Dexcom G6 continuous glucose monitor (CGM).
There were three FDA approvals of artificial pancreas systems on the market: The Medtronic 670G, approved in 2016, the Tandem Control-IQ™, approved in 2019, and the Medtronic 770G, approved in 2020. This marks the first tubeless hybrid closed loop system to receive FDA authorization.
Safe and effective system use was demonstrated in preschool children aged 2+ with T1D during a 3-month pivotal study. This year, we reported on the extension study, to evaluate if glycemic outcomes continued at 12 months, presented by Daniel DeSalvo, M.D., who had a Breakthrough T1D postdoctoral fellowship from 2014-2016 with world-renowned researcher Bruce Buckingham, M.D., at the American Diabetes Association’s 82nd Scientific Sessions. At 12 months, these children had lower A1c and greater time-in-range, and there was no DKA or severe hypoglycemia, indicating the potential long-term benefit of the Omnipod 5 in very young children with T1D.
For more information on it’s use for 6+, visit our blog, FDA Authorizes a Fourth Artificial Pancreas System.
Breakthrough T1D Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with increasingly advanced versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with type 1 diabetes have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The Breakthrough T1D Artificial Pancreas Project and the Breakthrough T1D Artificial Pancreas Consortium have dramatically accelerated progress by bringing academic researchers, government agencies, industry, and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
Breakthrough T1D has funded over $140 million to date in artificial pancreas research.
- Breakthrough T1D supported work in this area through investigators in the Breakthrough T1D Artificial Pancreas Consortium, Francis (Frank) Doyle, Ph.D., Eyal Dassau, Ph.D., and Howard Zisser, M.D., and their colleagues at the Sansum Institute (California). They created the first algorithm, which was eventually licensed by Insulet Corporation that led to the development of Omnipod 5.
- Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system in 2016, the first approved artificial pancreas system.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
For individuals who use the Tandem t:slim X2™, bolusing from their insulin pumps just got a bit easier.
On February 16, 2022, Tandem announced the FDA clearance of bolus insulin dosing from their t:connect® mobile app. Soon, individuals with the Tandem t:slim X2™ pump will be able to bolus remotely from either their iOS or Android smartphone. For people who keep their insulin pump in hard to access places, it’s a big deal.
“This is a gamechanger for me personally,” says Alecia Wesner, who lives with T1D. “I often wear clothing without any easily accessible places to keep my pump. That means I often store my pump on my bra or in my tights—which makes it hard to make the many daily boluses required to keep my blood sugar in range. This feature is simply going to make it easier for me to live with type 1 diabetes.”
Better Devices, Better Outcomes
People with T1D need better tools if they’re going to do better—that means achieving an optimal HbA1c, increased time-in-range, and more. This clearance makes the t:slim X2™ a better tool.
The t:slim X2™ with Control-IQ™ technology is an automated insulin delivery (AID), or artificial pancreas, system. This system is comprised of a Dexcom G6® continuous glucose monitor (CGM), the t:slim X2™ pump, and Control-IQ™ technology, which is the algorithm that automatically administers insulin on behalf of the user. AID systems have demonstrated they improve outcomes for people with T1D—but there are still barriers to adoption. These include ease of wear, comfort, and user interface.
This new feature potentially removes a barrier to adoption, meaning that more people will hopefully take advantage of this life-changing technology.
“Breakthrough T1D supports AP systems not just for their clinical benefits, but also for their ability to improve quality of life,” says Jonathan Rosen, Ph.D., Breakthrough T1D Associate Director, Research. “The FDA clearance of bolus insulin dosing on the t:slim X2 pump via the t:connect mobile app is great for the T1D community, since people will now be able to conveniently and discreetly bolus insulin from both iOS and Android smartphones.”
A Long History of Breakthrough T1D Support
Supporting research into better therapies for T1D has been a Breakthrough T1D research priority for decades. Breakthrough T1D founded the Artificial Pancreas Project in 2005 and the Breakthrough T1D Artificial Pancreas Consortium, which have dramatically accelerated progress by bringing academic researchers, government agencies, industry, and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
In addition to playing a leadership role in the field, Breakthrough T1D funded grants that supported the initial development of the Control-IQ™ technology, worked with the FDA to establish a regulatory protocol for AID systems like the t:slim X2™ with Control-IQ™, and, crucially, tirelessly advocated for the Special Diabetes Program (SDP), which funded the clinical trial that provided data for the 2019 FDA approval of Control-IQ™ .
While this is a notable step forward, Breakthrough T1D will continue working to improve therapy options for the T1D community.
Tandem plans for a limited rollout of this feature in the spring with an expanded release in the summer.
The Food and Drug Administration (FDA) authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system for individuals 6 and older. It includes an algorithm placed in a waterproof, closed loop insulin pump and communicates directly with a Dexcom G6 continuous glucose monitor (CGM).
There were three FDA approvals of artificial pancreas systems on the market: The Medtronic 670G, approved in 2016, the Tandem Control-IQ™, approved in 2019, and the Medtronic 770G, approved in 2020. This marks the first tubeless hybrid closed loop system to receive FDA authorization.
Data for Insulet’s submission of Omnipod 5 came from their Omnipod 5 Automated Insulin Delivery System pivotal trial. The trial included 128 adults and adolescents (14–70 years old) and 112 children (6–<14 years old) and demonstrated improvements in several key metrics, including a remarkably low rate of hypoglycemia:
- Adults and adolescents:
- Time in range (70–180 mg/dL) increased 9% from 65% to 74% compared to participants’ standard therapies (an additional 2.2 hours per day)
- Average HbA1c decreased from 7.16% to 6.78%
- Median time below 70 mg/dL decreased from 2.0% to 1.1%
- Children:
- Time in range (70–180 mg/dl) increased 15% from 53% to 68% compared to participants’ standard therapies (an additional 3.7 hours per day)
- Average HbA1c decreased from 7.67% to 6.99%
- Median time below 70 mg/dL remained at 1.5%
This data demonstrates that this system will help people with T1D achieve improved glucose control—a key goal of Breakthrough T1D’s Improving Lives program.
Breakthrough T1D Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with increasingly advanced versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with type 1 diabetes have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The Breakthrough T1D Artificial Pancreas Project and the Breakthrough T1D Artificial Pancreas Consortium have dramatically accelerated progress by bringing academic researchers, government agencies, industry, and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
Breakthrough T1D has funded over $135 million to date in artificial pancreas research.
- Breakthrough T1D supported work in this area through investigators in the Breakthrough T1D Artificial Pancreas Consortium, Francis (Frank) Doyle, Ph.D., Eyal Dassau, Ph.D., and Howard Zisser, M.D., and their colleagues at the Sansum Institute (California). They created the first algorithm, which was eventually licensed by Insulet Corporation that led to the development of Omnipod 5.
- Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system in 2016, the first approved artificial pancreas system.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
Per Insulet, “Omnipod 5 is expected to be broadly available shortly after the limited market release.”
Hybrid closed loop or artificial pancreas systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an algorithm that automatically administers insulin. And a new paper published in the New England Journal of Medicine demonstrates that hybrid closed loop systems help children as young as 1 year old achieve better glycemic control.
This study, led by Roman Hovorka, Ph.D., and funded in part by Breakthrough T1D, compared data from children ages 1-7 on hybrid closed loop therapy to a CGM and insulin. The data showed conclusively that the children on the hybrid closed loop therapy did better.
Breakthrough T1D has funded over $135 million in artificial pancreas research to date, in addition to working tirelessly to ensure these systems have a reasonable pathway to regulatory approval and are covered by payers.
More Proof Artificial Pancreas Systems Work
There have been many, many studies examining the benefits of artificial pancreas systems on glycemic control in a myriad of patient populations. These studies, several of which have been funded by Breakthrough T1D, have led to the development and commercialization of multiple systems with more on the immediate horizon.
This study is yet another to show the benefits. Children on the hybrid closed loop system spent an average of 8.7 percent more time-in-range each day compared to the control group. That’s over two hours every day! Children on the system also had lower HbA1cs and without increased hypoglycemia.
Artificial Pancreas Systems: What’s Available?
Led by Breakthrough T1D, the past 20 years have seen a flurry of innovation and investment in artificial pancreas research. As this technology continues to advance, more patient populations will have access to and benefit from these therapies. In Europe, CamAPS—the artificial pancreas system in this publication—is approved for ages 1 and up and the Medtronic 780G is approved for users between ages 7 and 80 years old. In the United States, the Medtronic 770G is the only automated insulin delivery system approved for children as young as two, and the Tandem Control-IQ™ is approved for individuals 6 and up. (There is a Control-IQ™ clinical trial currently recruiting in the United States, studying these systems in children ages 2-6.)
Type 1 diabetes (T1D) and sleep, or lack thereof, are often tied together. Someone living with T1D must navigate a wide range of challenges that can impact sleep, and poor quality of sleep negatively affects your psychological and physical health.
In addition, one of the greatest fears of nighttime is low blood sugar (called hypoglycemia), which occurs when blood-sugar levels fall below 70 mg/dL. Symptoms can include shaking, an accelerated heart rate, and clammy skin. It’s possible to sleep through a hypoglycemic event, but you can wake up tired, confused, sweaty, and can feel lethargic or foggy.
But artificial pancreas systems are changing that.
Artificial pancreas systems (also called automated insulin delivery systems) have two interrelated functions: Monitoring the blood-sugar levels in your body (through a continuous glucose monitor) and automatically providing insulin to keep them in a healthy range (through an insulin pump), via a smart algorithm. By automating much of blood-sugar management, these systems can help improve diabetes-related outcomes and lighten the burden of T1D.
Even during sleep.
Time-in-Range and Sleep
Time-in-range (TIR) is a measurement that tells you what percentage of the day your blood sugars are in your goal range (typically 70-180 mg/dl). The American Diabetes Association recommends that people with diabetes spend 70% of their time in the target range, meaning you should aim for roughly 17 out of 24 hours each day to be in range. Unfortunately, less than half of the diabetes population meets this recommendation.[i]
But experts emphasize that even a 5% improvement in TIR—let’s say, going from 60% to 65%—is meaningful, as it translates to one more hour per day spent in-range.[ii]
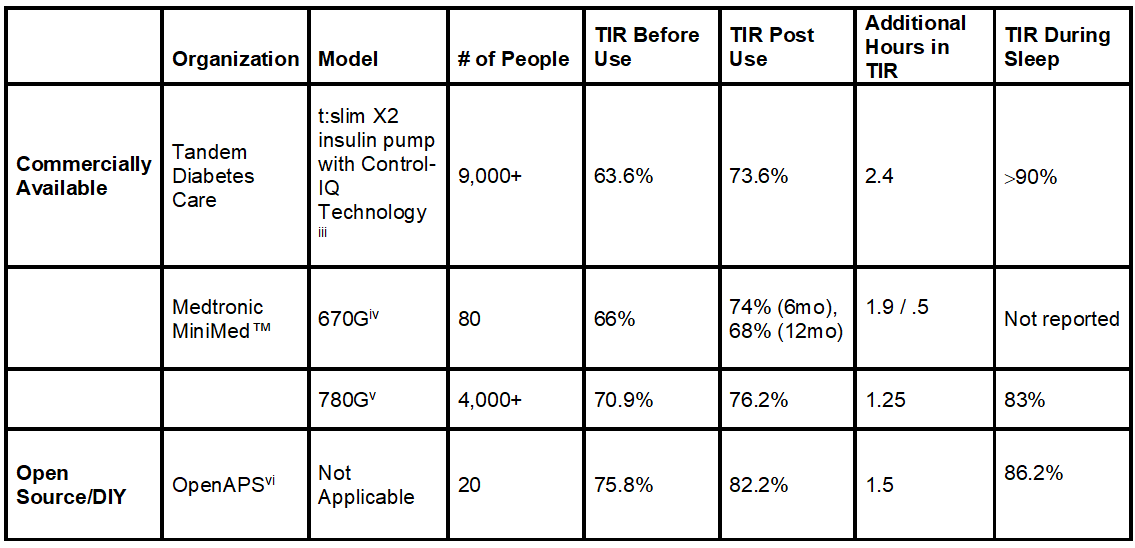
Now, let’s look at some recent scientific findings in the table below.
Not only can the artificial pancreas systems improve time-in-range, but they can dramatically improve time-in-range during sleep.
Note: Left click on image to enlarge.
More time-in-range, even when you’re asleep. Now that’s research that can help you rest easy and more often than not, wake up on the right side of the bed.
Have a goodnight!
Watch Breakthrough T1D’s and Tandem’s Facebook Live event (video below) exploring AP systems and improvements in TIR and sleep.
Editor’s Note: This educational content is made possible with support from Tandem Diabetes Care. Breakthrough T1D produces this content to provide information to our supporters about their potential options for managing their T1D and not as an endorsement of products. Editorial control rests solely with Breakthrough T1D.
RX ONLY. The t:slim X2 insulin pump with Control-IQ technology is indicated for patients with type 1 diabetes, 6 years and older. BOXED WARNING: Control-IQ technology should not be used by people under age 6, or who use less than 10 units of insulin/day, or who weigh less than 55 lbs. For full safety information, visit tandemdiabetes.com/safetyinfo.
[i] Bergenstal RM, Hachmann-Nielsen E, Tarp J, Kvist K, Buse JB. 65-LB: Real-World Continuous Glucose Monitoring Data on Time-in-Range from a U.S. Population, 2015–2019. Diabetes Jun 2021, 70 (Supplement 1); doi: 10.2337/db21-65-LB
[iii] Breton MD, Kovatchev BP. One Year Real-World Use of the Control-IQ Advanced Hybrid Closed-Loop Technology. Diabetes Technol Ther. 2021; 23 (9): 601-608. doi:10.1089/dia.2021.0097
[iv] DuBose S, Bauza C, Verdejo A, Beck RW, Bergenstal RM, Sherr J. Real-world, Patient-Reported and Clinic Data from Individuals with Type 1 Diabetes using the MiniMed 670G Hybrid Closed Loop System. Diabetes Technol Ther. 2021; 10.1089/dia.2021.0176. doi:10.1089/dia.2021.0176
[vi] Lewis DM, Swain RS, Donner TW. Improvements in A1C and time-in-range in DIY closed-loop (Open-APS) users. Diabetes. 2018; 67: 352-OR. https://diabetes.diabetesjournals.org/content/67/Supplement_1/352-OR.abstract.
One of the largest pain points (literally) for people with type 1 diabetes (T1D) is difficulties with infusion sites. They can be uncomfortable, become infected, and need to be changed at least every three days—until now.
The U.S. Food and Drug Administration (FDA) has approved Medtronic’s Extended Wear Infusion Set (EWIS), which is the first infusion set approved for seven days. The EWIS is approved for a significantly longer duration; no other infusion set is currently approved for more than 3 days.
“Infusion sets that last longer, perform better, and are more comfortable to wear are critically important to creating better devices to manage type 1 diabetes,” said Breakthrough T1D Vice President of Research, Sanjoy Dutta, Ph.D. “Breakthrough T1D applauds the FDA for approving Medtronic’s EWIS, which has demonstrated significant benefits that will help people with type 1 diabetes achieve better glycemic control and improve their quality of life.”
EWIS data was presented at the American Diabetes Association 81st Scientific Sessions (listen to Breakthrough T1D CEO Aaron Kowalski, Ph.D., discuss it in his Happy Hour), showing the EWIS is safe and performs better than 2-3 day sets. Aside from the 7-day wear, other positive data include:
- Improved glycemic outcomes for people wearing the EWIS; and
- Increased survival compared to 2-3 days sensors (74.7% to 67.7%).
Breakthrough T1D’s improving lives portfolio is committed to making it easier for people with type 1 diabetes to achieve ideal blood-glucose levels—which includes making wearable devices smaller, better, and more comfortable. Breakthrough T1D has funded several million dollars in research grants to address this specific issue. This includes our initiative on “Extended Wear Infusion Sets” that has funded several projects to unlock the potential of longer lasting disposables. One example is Breakthrough T1D’s partnership with Becton Dickinson to create an extended wear infusion set to be worn for more than 3 days, that was also supported by the Helmsley Charitable Trust. The Breakthrough T1D T1D Fund is an investor in Capillary Bio, a biomedical company developing a novel 7-day infusion set. Learn more about Breakthrough T1D’s efforts in improving lives here.
Learn more from Medtronic.