The “who” of clinical trials
People with type 1 diabetes (T1D) who choose to participate in clinical trials are central to determining whether a new treatment is effective and safe. Yet have you ever wondered who operates behind the scenes of clinical trials?
There are scientists that have researched a new drug or device for years, sometimes decades. There are healthcare professionals (HCPs) that oversee the trial at each location, administer treatments, and monitor participants’ health. There are study coordinators that manage the volunteers. There are pharmacologists, biostatisticians, ethicists, writers, and countless others who analyze and publish the data for the scientific community. It takes a village!
Focus on Hassenfeld Children’s Hospital at NYU Langone
The Pediatric Diabetes Center at Hassenfeld Children’s Hospital at NYU Langone is part of the world-renowned NYU Langone Health system, which also has adult programs for diabetes. Diamyd Medical specifically chose NYU Langone as a trial site for DIAGNODE-3.
DIAGNODE-3 is a Breakthrough T1D-funded phase 3 clinical trial of the disease-modifying therapy Diamyd®. The goal is to preserve as many healthy beta cells as possible to maintain the body’s own insulin production.
The team at Hassenfeld Children’s Hospital managing DIAGNODE-3 is led by Mary Pat Gallagher, MD, the Principal Investigator, Director of the Pediatric Diabetes Center, and associate professor in the Department of Pediatrics at NYU Grossman School of Medicine. With many years of experience under her belt, she has been involved in previous clinical trials for Diamyd®, making her a perfect fit for this final stage of testing.
The team also includes pediatric endocrinologist Dr. Casey Berman, the Sub-Investigator, in addition to Jeniece Ilkowitz, RN, CDCES, and Aashna Shah, who are the study coordinators.
I have a personal and family connection to T1D, and research allows me to have both an impact on the greater community as well as individuals.”
Managing T1D is a team effort—the clinical researchers behind the scenes want to make the process as smooth as possible for those who choose to participate in trials.
“We have a wonderful research team with people who also live with diabetes,” Jeniece continued. “You will receive extra support as you learn to live with your new diagnosis, as we will support you as well as communicate with your primary diabetes provider.”
A deeper dive into the science
Diamyd® is an immune modulator that prevents attack on the body’s insulin-producing beta cells by reprogramming the immune system to ignore GAD (glutamic acid decarboxylase). In T1D, GAD can give rise to autoantibodies, which tell the immune system to destroy beta cells.
This therapy is targeted toward people with newly diagnosed T1D who also have the genetic marker associated with GAD —about 40% of the T1D population.
Diamyd® works by injecting GAD directly into lymph nodes, where immune cells live until they’re needed. If certain types of immune cells are exposed to GAD over and over again, they eventually become tolerant and stop destroying the beta cells.
DIAGNODE-3: The phase 3 trial
Researchers are now testing Diamyd® in phase 3 clinical trials (DIAGNODE-3), the final step before seeking approval from the U.S. Food and Drug Administration (FDA). Results from previous trials have been so promising that Diamyd® has received Fast Track Designation and Orphan Designation from the FDA, solidifying it as a potentially life-changing disease-modifying therapy for newly diagnosed T1D.
To participate in DIAGNODE-3, you must be between 12-28 years old and have been diagnosed with stage 3 T1D within the last six months, meaning you have symptoms associated with high blood sugar and require insulin therapy. You also must test positive for a specific genetic marker (HLA DR3-DQ2) to participate.
Over a two-month period, participants will receive three ultrasound-guided injections into a lymph node located in the groin, followed by a 22-month follow-up. A topical anesthetic can be used as required. While a mild reaction at the injection site (itching, redness, etc.) is expected, to date none of the participants in all the trial phases has discontinued treatment due to injection issues.
For every two participants receiving Diamyd®, one will receive a placebo (a treatment with no therapeutic effect for comparison). Researchers will measure C-peptide, a lab test that shows a person can still produce their own insulin, in addition to HbA1c and continuous glucose monitor (CGM) data to determine whether the drug is working. Based on previous trials, researchers predict that Diamyd® will significantly improve blood glucose management, reduce risk of complications, and overall increase quality of life.
We need your help—get involved!
Clinical trials are critical to get new medicines from the lab into the clinic in a safe and effective way. We know that trials are daunting—signing up to receive a treatment that’s still under investigation can be a difficult decision. Yet, if you choose to participate in a clinical trial, you can have access to new therapies and knowledgeable clinicians, learn more about T1D, and benefit the broader T1D community. Also, because there are only 13 DIAGNODE-3 clinics in the U.S., you may be eligible to receive travel reimbursement.
Meet the team and get screened!
Still feeling unsure? Jeniece, one of the study coordinators at Hassenfeld Children’s Hospital at NYU Langone, suggests starting simple.
“As you wait for the results, you can speak to family and friends with the added knowledge as well as think about your life and how this can fit into it,” she explained. “We truly want to help people living with diabetes and be able to have as many options as possible to help them manage their disease in the future.”
Learn more about DIAGNODE-3 by visiting the study’s website. While there are 60 clinic locations in the U.S. and Europe and more than 200 people with T1D enrolled so far, only one person has enrolled at NYU Langone. If you or anyone you know in the NY Metro Area would be interested in participating, please contact the research team at NYU to learn how to get involved. They are recruiting throughout 2025.
Breakthrough T1D’s role
A pillar of Breakthrough T1D’s Cures Program is disease-modifying therapies: those that can slow, halt, or reverse the course of T1D, such as Diamyd®. These interventions are most impactful at the earlier stages of T1D with the hopes of preserving as many beta cells as possible so that the body can produce its own insulin.
Breakthrough T1D’s funding of the DIAGNODE-3 clinical trial aligns with our dedication to early detection and screening. Identifying the presence of T1D autoantibodies as early as possible allows for a larger window of intervention and increases the chances of preserving healthy beta cells with disease-modifying therapies. While DIAGNODE-3 is the first therapy to target a specific genetic subpopulation of T1D, we anticipate that it won’t be the last, which underscores the importance of getting screened to understand your genetic T1D background.
DIAGNODE-3 is just one of the many different clinical trials that Breakthrough T1D funds.
To promote education and awareness of clinical trials, Breakthrough T1D has assembled a group of Clinical Trial Education Volunteers (CTEVs) who engage with the T1D community.

One CTEV, Alecia Wesner, used her expertise and personal experience with T1D to connect Diamyd Medical, the NYU Pediatric Diabetes Center, the Breakthrough T1D NY Metro Chapter, and Breakthrough T1D National, bringing together critical players who can facilitate participation and spread the word about the DIAGNODE-3 clinical trial.
Without people like Alecia, getting the message out about potentially life-changing trials would be far more difficult. We thank Alecia for her efforts and the clinical trial participants of the T1D community for moving science forward for the benefit of all.
Today, Sana Biotechnology released significant clinical data: the first person with type 1 diabetes (T1D) who received deceased donor islets engineered to evade the immune system is producing insulin without immunosuppression.
The details
This is a big step for cell-based therapies for T1D. Sana’s first-in-human study consists of allogeneic islets, meaning they are derived from an external source, which in this case is the pancreases of deceased donors. These islets were engineered to avoid recognition by the immune system (hypoimmune) and were implanted intramuscularly into a person with T1D. After four weeks, circulating C-peptide increased, meaning that the beta cells are alive, healthy, and producing insulin—all without the need for immunosuppression and no safety issue. This is the first evidence of engineered islets successfully avoiding immune destruction.
What this means for the T1D community
While this is an incredibly promising step forward for the T1D community, currently available cell therapies that rely on deceased donor islets (Lantidra®) are only accessible to a small portion of the T1D population because there are very few donor cells available. They also require broad anti-rejection immunosuppressants, which can come with serious side effects that may not be manageable for everyone with T1D. Engineering cells to evade immune attack is a new path forward to protect the insulin-producing beta cells and avoid the use of immunosuppressants. Most importantly, this technology can now be applied to stem cell-based therapies, which is a scalable solution for many people with T1D.
What’s next: lots to look forward to
Breakthrough T1D believes that the best bet for type 1 diabetes cures lies in stem cell-based therapies since deceased donor islets are in short supply, while stem cell-derived islets can be produced at scale. We have now opened the doors to apply hypoimmune technology to stem cell-derived islets, moving us closer to the possibility of having enough immune-evading cells for everyone with T1D. While this will take significant time, effort, and money, every day we take another step toward a possible life-changing T1D cure.
Breakthrough T1D’s role
The primary objective of Breakthrough T1D’s beta cell replacement efforts is to place insulin-producing cells into people with T1D without the use of immunosuppressants. Breakthrough T1D strongly supports the development of stem cell-based therapies that do not require broad immunosuppression and recently launched an initiative to accelerate this faster than ever (Project ACT – Accelerate Cell Therapies). To contribute to the advancement of these game-changing therapies, the T1D Fund: A Breakthrough T1D Venture invested in Sana recognizing that their hypoimmune engineering technology held significant promise for type 1 diabetes cell therapies. We look forward to seeing how the trial progresses.
While we look back on 2024, we can reflect upon the incredible progress we’ve made in advancing breakthroughs toward cures and improving everyday life with T1D.
This wouldn’t have been possible without each and every one of you and your continued support of our mission as we drive toward cures for T1D.
Here are the top 11 advances that together, we made happen in 2024:
Breakthrough T1D announced the launch of Project ACT, an initiative aimed at accelerating breakthroughs in T1D cell replacement therapies that do not require broad immunosuppression. Recent advances, such as Vertex’s stem cell-derived islets, have been made possible by Breakthrough T1D’s Cell Therapies program as part of our drive toward cures. The goal of Project ACT is to push research, development, regulatory policies, access, and adoption to increase the rate at which cell therapies without the need for broad immunosuppressants will become available to people with T1D.
Why this matters: Immunosuppressive drugs are a barrier to access to cell replacement therapies because of their toxic side effects, which is why islet transplants are currently only available to people with severe low blood sugar (hypoglycemic) unawareness and episodes. By striving toward a future where we realize the benefits of cell replacement therapies without the downsides of the current regimen of immunosuppressants, we will make islet replacement therapies broadly accessible to the T1D community.
Vertex’s clinical trial of VX-880, a first-generation stem cell-derived islet replacement therapy for people with severe hypoglycemia (requiring the use of immunosuppressants), has transitioned into a phase 1/2/3, or pivotal, trial. This news comes after Vertex shared incredibly promising data in the earlier phases of the trial, including 11 of 12 participants reducing or eliminating the need for external insulin.
The upcoming trial will expand to 50 people who will get a single, target dose of VX-880. The primary endpoint will be insulin therapy independence without severe hypoglycemic events after one year. This is the final clinical testing stage before Vertex can seek FDA approval.
Breakthrough T1D has a decades-long relationship with Vertex and the leading scientists behind stem cell-derived islet therapies, an advancement that would not have been possible without Breakthrough T1D funding and support. The T1D Fund had invested in Semma Therapeutics, which was acquired by Vertex Pharmaceuticals in 2019, eventually leading to the active clinical development of VX-880 in T1D.
Why this matters: This is the first time a scalable cure for T1D is entering phase 3 clinical trials—a significant win and a huge step toward accelerating the delivery of cell therapies to members of the T1D community!
Tegoprubart: Transplant Survival Without Standard Immunosuppressive Drugs
Tegoprubart, an anti-CD40L immunotherapy that limits the immune response, is being tested in a Breakthrough T1D-funded study in people with T1D and severe hypoglycemia who have received deceased donor islets. Eledon Pharmaceuticals announced promising initial results in which two of three people achieved insulin therapy independence. According to the study, tegoprubart is safer for both people and transplanted cells in comparison to broad immunosuppressants, with milder side effects and greater islet survival. To further support this effort, the T1D Fund: A Breakthrough T1D Venture invested in Eledon.
Cell Pouch: A Home for Transplanted Islets
Breakthrough T1D has been supporting the development of Cell Pouch, an implantable device from Sernova that provides a safe, immune-protected environment for transplanted islet cells. In phase 1/2 clinical trials, all six people who received donor islets within the Cell Pouch achieved sustained insulin therapy independence with immunosuppressants, including long-term islet survival and function over five years without harmful side effects.
Why this matters: Standard of care immunosuppressive drugs that help avoid transplant rejection come with unwelcome side effects, such as increased risk of infection and malignancy and toxicity to kidneys, nerves, and islet cells themselves. Breakthrough T1D is focused on finding alternative ways to keep transplanted islet cells alive and healthy so that cell replacement therapies can become more tolerable and accessible.
In a major effort spearheaded by Breakthrough T1D, the first internationally recognized clinical guidelines for those who test positive for T1D autoantibodies have been published. These include guidance on monitoring frequency, education, and psychosocial support in addition to recommended actions for healthcare professionals (HCPs) when the risk of T1D progression is high. The guidelines were cooperatively developed with over 60 international experts spanning ten countries.
Why this matters: Previously, there had been no consensus on monitoring guidelines for people who test positive for T1D autoantibodies. Standardization of clinical recommendations means that individuals, families, and HCPs have tangible next steps to monitor early T1D progression and catch life-threatening complications sooner.
- Breakthrough T1D is leading a campaign to secure a recommendation for T1D screening from the U.S. Preventative Services Task Force (USPSTF), the main authority for preventative care. Approval would require T1D screening to be covered by insurance—an important step forward in expanding access.
- Driven by Breakthrough T1D’s advocacy efforts, The Centers for Medicare and Medicaid Services (CMS) established a unique ICD-10 code for stage 2 T1D. ICD-10 codes are used by HCPs to classify and document diagnoses, symptoms, and procedures. These codes provide a unified way for doctors and providers to indicate what diseases or conditions a person has in their electronic health record (EHR), empowering HCPs to document accurate diagnoses and provide the best possible care.
Why this matters: T1D early detection is critically important to prevent life-threatening complications at diagnosis and to give people necessary resources to make informed decisions about their health. Integrating T1D screening into the U.S. healthcare system will increase access to care.
The past year has seen some important advances in glucose management therapies and devices:
- Cadisegliatin, an activator of a blood sugar regulator in the liver, is being investigated in a phase 3 clinical trial (TTP399) as an adjunct therapy to insulin for people with T1D, although it is currently placed on clinical hold. vTv Therapeutics, the trial sponsor, is also a T1D portfolio company.
- The Omnipod 5 app is now available for the iPhone, making it easier to control the Omnipod without the need to carry a controller. It can also integrate with the Dexcom G6 continuous glucose monitor (CGM).
- Eversense 365 is the first FDA-approved year-round sensor that can easily integrate with automated insulin delivery (AID) systems. Other sensors require replacement after 10-14 days.
Why this matters: While advancements in glucose management have been pivotal in improving health outcomes for people with T1D, access remains a challenge. AID systems are globally underutilized, and not everyone has the necessary technology to connect devices. Breakthrough T1D is working to not only support advances in glucose management but also increase access.
Related content: While Breakthrough T1D consistently strives to improve the lives of those living with T1D, as an organization we have made incredible progress in the development of AID systems, also called the artificial pancreas systems. Read a historical perspective written by Breakthrough T1D volunteer Doug Lowenstein that covers conception to FDA approval of the first artificial pancreas systems, which changed the lives of people with T1D.
An inquiry spearheaded by the Breakthrough T1D affiliates in the U.K. uncovered risks of developing T1D eating disorders (T1DE), including bulimia, anorexia, or insulin restriction to lose weight. There is a significant gap in education and clinical guidelines for HCPs, a lack of internationally recognized criteria for T1DE diagnosis, and insufficient care integration, leading to preventable complications and healthy years of life lost. Breakthrough T1D recognizes the importance of spreading awareness and support for T1DE, and much work is needed to improve the lives of those living with T1DE.
Why this matters: There is an urgent need to change the way T1DE is approached, including integrated physical care with mental health services to get people with T1DE the access to care that they need.
In a study that included people with T1D, finerenone (Kerendia®) has been shown to improve cardiovascular outcomes in adults with heart failure. The drug is already approved in the U.S. to treat kidney and cardiovascular disease in people with T2D. Based on these results, Breakthrough T1D is supporting a clinical trial (FINE-ONE) in conjunction with Bayer to investigate the use of finerenone for T1D with the hopes of reducing kidney complications.
Why this matters: Kidney and cardiovascular disease remain significant challenges for those with T1D, especially given the FDA’s recent rejection of an SGLT inhibitor to lower blood glucose in people with T1D and chronic kidney disease. Yet, a new clinical trial (SUGARNSALT) will better assess the benefits versus risks.
Breakthrough T1D is advocating for the regulatory approval of C-peptide, a biomarker for insulin production by beta cells, to be used as an endpoint in clinical trials. An endpoint can accurately predict a meaningful benefit in clinical trials for disease-modifying therapies (DMTs; treatments that can slow, halt, or reverse T1D). To support this endeavor, Breakthrough T1D scientists and an expert consensus panel published research with evidence supporting C-peptide as an endpoint. Breakthrough T1D is continuing to engage with regulators, coordinate with industry, and assess more clinical trial data to drive this effort forward.
Why this matters: Current clinical trial endpoints (HbA1c, hypoglycemia, and complications) are not the best way to gauge the clinical benefits of T1D therapies. If C-peptide gets regulatory approval to be used as an endpoint, clinical trials could be smaller and shorter while still accurately assessing the advantages of a DMT. This means that drug development can move more quickly, and people with T1D will be able to access therapies sooner.
Related content: Two years ago, the T1D community received the incredible news that Tzield® had become the first FDA-approved disease-modifying therapy that can significantly delay T1D onset. Breakthrough T1D volunteer Doug Lowenstein recounts the life-changing drug’s journey nearly 100 years after the discovery of insulin.
The T1D Index is a data simulation tool that measures the global health impact of T1D, bridging gaps in our knowledge of public health statistics. T1D Index 2.0 has new and improved functionality, including advanced simulation capabilities, validation of data, and enhanced user experience. Breakthrough T1D contributed to both the development and improvement of the T1D Index.
Why this matters: The T1D index is critical in defining the intercontinental scope of T1D, driving us toward country-specific solutions and improved global health outcomes.
Earlier this year, JDRF rebranded to Breakthrough T1D. While our mission remains the same, our name needs to better reflect who we are and where we’re going. Our new brand aligns with our mission to accelerate life-changing breakthroughs for those of every age living with T1D as we work toward a world without it.
Why this matters: The proof is in the name—each day we strive to increase and accelerate breakthroughs in T1D, and it’s critical for our brand to accurately reflect our mission.
It’s certainly been an exciting year! While we still have more work to do, it’s crucial to celebrate our wins, both big and small, to see how far we’ve come in our push to make T1D a thing of the past.
Together, we’re accelerating breakthroughs for people with T1D, and the support of the T1D community drives our mission forward every single day, leading the way to lifechanging therapies and cures. Let’s see what 2025 has in store!
Aiedan Mosley, a 16-year-old San Francisco native with autism spectrum disorder, enjoys football, track and field, music, and movies. He can play tunes by ear and loves Journey and old-school rap. Aiedan aspires to be a musician, actor, and film producer, like his role model Ice Cube. “Aiedan has worked so hard to be where he is today, and we are so proud of all the hard work,” said his caregiver, Jessica.
Typical high school life changed in June of 2024. Jessica noticed differences in Aiedan’s behavior, including mood swings, tiredness, and weight loss. Worried, she scheduled a visit at the University of California San Francisco (UCSF) Adolescent Health Clinic. The day before, Aiedan was so thirsty that he drank four glasses of water before dinner. After the appointment, a call from the doctor revealed that Aiedan’s blood glucose was 550 mg/dL—they immediately rushed to the emergency room.
“Aiedan was admitted for 3 days, and we got a quick tutorial into the world of type 1 diabetes (T1D). Nothing can prepare you or your child for the finger pokes and the insulin shots,” explained Jessica. The diagnosis came as a shock since they had no family history of T1D.
Soon after, Jessica started researching clinical trials, curious to know if there was a way to preserve Aiedan’s insulin production. Feeling unsure, she spoke with Breakthrough T1D for additional support and had an honest conversation with Aiedan. “He said he wanted to make a difference and find a cure,” said Jessica. Aiedan agreed to participate in TrialNet’s T1D RELAY Study.
TrialNet’s T1D RELAY Study: A new combination of two FDA-approved immune therapies
TrialNet, the largest T1D clinical trial network in the world, is aimed at preventing T1D and stopping disease progression by preserving insulin production before and after diagnosis. TrialNet is recruiting people newly diagnosed with T1D for a clinical trial (T1D RELAY) that will evaluate the combination of two FDA-approved immune therapies, rituximab-pvvr (Ruxience®) and abatacept (Orencia®), in maintaining insulin production. Both are FDA-approved for other autoimmune diseases such as rheumatoid arthritis.
Stemming from earlier TrialNet studies in which each therapy alone delayed insulin loss, this trial will assess whether abatacept given after rituximab-pvvr will better preserve insulin production compared to rituximab-pvvr alone. Because this strategy has shown promise in rheumatoid arthritis, researchers are confident that this may be a successful approach for T1D.
“Clinical trials are how we find cures”
Aiedan is handling the trial like a superstar. He experienced mild side effects from rituximab-pvvr, but his biggest challenge was keeping up with missed schoolwork because of lengthy infusion days. Now, Aiedan is receiving either abatacept or a placebo at home and has had few hyperglycemic events since the start of the trial.
For families interested in exploring clinical trials, Jessica’s advice is to do your research and be realistic with your child. “Aiedan listened to me and the [trial] coordinator, and he made the choice to move forward with the study knowing it could be hard at times; in the end, it will benefit not only him but many others,” said Jessica. “Clinical trials are how we find cures.”
Support the T1D community by participating in clinical trials
Clinical trials are key to pushing medical advancements forward so we can get therapies to people with T1D quickly and safely. By choosing to participate in a clinical trial, you can directly impact the T1D community by helping to accelerate treatments and cures.
Click here to learn more about clinical trials.
Table of Contents
Author’s Note
Chapter 1: The Beginning: A Parent
Chapter 2: The Scientist
Chapter 3: The Patient Organization: Cure or Treat
Chapter 4: “Now What Do We Do?”
Chapter 5: The Big Gamble
Chapter 6: The Device Makers
Chapter 7: The Inventor
Chapter 8: The Tide Turns
Chapter 9: The Allies
Chapter 10: The Regulators
Chapter 11: The Hackers
Chapter 12: The Patients
Chapter 13: The Finish Line
Chapter 14: The Aftermath
Postscript
Author’s Note
In April 2001 our younger daughter Emma was diagnosed with T1D at age 14. We quickly found our way to JDRF and dove into working with our local chapter in Washington, DC. We were inspired when we heard there would be a cure in five years. We got excited about islet cell transplants only to realize that they would never be of use to Emma. We got excited about every trial that cured T1D in mice. But as the years dragged on, we watched and helped Emma struggle to manage T1D and we ached for her. We started to drift away from JDRF because we no longer believed that JDRF’s singular focus on a cure would help Emma. What was it doing to keep her healthy until a cure was found? Along the way, we met kindred and similarly frustrated and impatient spirits like Jeffrey Brewer. We heard this young new JDRF scientist Aaron Kowalski speak passionately about a new initiative he was leading called the Artificial Pancreas Project. And we were inspired anew.
In 2023, I wrote a story about the development of the first-ever T1D disease-modifying immune therapy, teplizumab. When I finished, I started thinking about other stories in the T1D field that deserved to be told. I quickly focused on the history of artificial pancreas development as a possibility. I realized that while others have written about it, the definitive history of this remarkable journey had yet to be written. In the pages that follow, I try to fill that void.
While this story focuses on a handful of people who played central roles in securing approval of AP systems, space does not allow me to highlight the instrumental contributions of hundreds of passionate scientists, Breakthrough T1D volunteers, clinical trial participants, industry executives and researchers, regulators, lawmakers, and government scientific agencies. While they may not receive the explicit recognition they deserve, what follows is their story as much as it is the story of those featured. They all helped make history, and helped people like my daughter live safer and healthier lives. Our family owes them a debt of gratitude we can never repay.
This story is based on more than 30 one-on-one interviews lasting a total of more than 40 hours, and review of hundreds of pages of peer-reviewed articles in scientific journals. I am responsible for the content of this story in its entirety. I hope you enjoy reading it.
Douglas Lowenstein
Washington, DC
November, 2024
Chapter 1: The Beginning: A Parent
It was 2002 and Jeffrey Brewer, a successful technology and Internet entrepreneur, was worried and frustrated. His son Sean had been recently diagnosed with type 1 diabetes (T1D). T1D is a burdensome autoimmune disease where a person’s immune system mistakenly destroys the insulin-producing beta cells that live in the pancreas.
“The doctors explained to me Sean would be dependent on a hormone called insulin,” Brewer recalled. “They told me the dangers of not dosing the drug correctly: too little and his blood sugar (or blood glucose) levels would be too high putting him at increased risk for long-term health complications; too much and he was at risk for low blood sugar with the potential of becoming unconscious and even dying if no one’s around to help. I was given some needles, insulin vials, a blood glucose meter1, and literally a handwritten sliding scale to calculate correct insulin doses throughout his day. I couldn’t understand why it was so rudimentary.”
Much of the food we eat contains carbohydrates, a form of sugar, or glucose, that our body converts to fuel. In healthy people, when the body absorbs sugar from carbs, it makes just the right amount of insulin to ensure that blood-sugar levels remain safe. But those with T1D are stripped of their capacity to make insulin, so they must execute a delicate dance where the amount of carbs they consume is precisely balanced by the amount of insulin they dose. Over a typical day, the individual with T1D, or their caregivers, must make dozens of complex calculations daily where a single mistake could be fatal. With the technology at hand when Sean was diagnosed, the task was virtually impossible to get it right for a day, let alone for weeks at a time.
Tall and lanky, Brewer was almost always dressed in khaki slacks, a button-down shirt with a school backpack over his shoulders. He was intense and restless, and a friend said, “you can always hear his mind clicking.” These characteristics explain why he was convinced there had to be a better way to manage T1D. And soon he gravitated to an organization he believed would help his son and others safely manage their disease.
The Juvenile Diabetes Research Foundation (JDRF)2 was a nonprofit founded in the 1970s by a group of parents dedicated to funding research to cure T1D. By the early 2000s, JDRF had poured money toward that goal over a 30-year period and while there were some promising advances no biological cure was in sight3.
As Brewer got more involved, he joined a committee of JDRF volunteers that reviewed grant applications brought forward by the research staff, where he had a chance to interact directly with leading T1D scientists. He and other committee members heard one proposal after another to carry out basic science experiments rather than projects that would directly lead to therapies helping people already living with T1D. His doubts about JDRF’s priorities began to grow.
“I realized very quickly that we weren’t anywhere close to a cure for T1D,” Brewer recalled. “In the meantime, my son was going to need something better than what was commercially available for a long time.” With this realization, Brewer had his mission—to keep Sean and others with T1D healthy so that when a cure arrived, they would be healthy enough to benefit. He also had the tenacity and business experience to pursue this goal.
[1] Blood glucose meters are handheld devices where the user pricks their fingertip with a needle that draws a small spot of blood that is then put on a strip and inserted into the machine to generate a glucose reading. The constant pricking can create scars and can be mildly painful.
[2] The organization was rebranded as JDRF in 2010 and in 2024 was again rebranded as Breakthrough T1D.
[3] Around this time, Canadian Dr. James Shapiro at the Alberta Diabetes Institute pioneered islet cell transplants, a procedure to harvest pancreatic cadavers, remove the islet cells where beta cells lived, and transplant them in people with acute T1D. Some people felt this was a major step toward a cure but numerous practical issues, including a limited supply of pancreatic cadavers and the need for recipients to take a lifetime of immune suppressive drugs with adverse side effects, limited its potential for most people with T1D.
Chapter 2: The Scientist
Aaron Kowalski was also frustrated.
Kowalski’s younger brother Steve had been diagnosed with T1D in 1977, and a few years later, in eighth grade, Aaron was diagnosed. “There was a lady who lived on our street fully blinded by T1D,” he recalled. “When we first met her, she had a cane and then she had a seeing eye dog. She was in her twenties. When I was diagnosed, I was like, ‘I just don’t want to go blind.’” Like Brewer, Kowalski was certain there had to be a better way to manage T1D and in his third year of undergraduate work at Rutgers University, he decided to become a biology major “with the purpose of going to graduate school to work in diabetes.” He would go on to earn his Ph.D in microbiology and molecular genetics, anticipating a career in the pharma industry.
He started working at a hospital in Newark, NJ, doing research on hypoglycemia (low blood sugar). But he wasn’t happy. “I was looking for post-doctorate jobs in various scientific journals and I saw an ad from JDRF seeking a scientific project manager to work on diabetes complications including hypoglycemia, exactly what I was doing. I literally typed up an email and sent it.”
In September 2004, JDRF hired him as a research scientist to lead its program to find ways to mitigate the long-term complications of T1D ranging from blindness to kidney and cardiovascular disease to neuropathy.
Kowalski knew that a landmark study (the Diabetes Control and Complications Trial, or DCCT) by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), a unit of the National Institutes of Health (NIH), had found that people with T1D could reduce the risk of long-term complications by 35-76% through extremely tight blood-sugar control—keeping their levels in a range that largely eliminated dangerous highs and lows. There was just one gigantic problem: achieving the DCCT ideal target blood-sugar levels was virtually impossible to do safely for even the most meticulous person living with T1D using the existing “rudimentary” technology.
“The people in the DCCT who had intensive management had an incredible amount of help during the trial 24/7,” said Judy Fradkin, who would eventually become director at NIDDK’s diabetes division. “Even knowing the huge impact it had on them during the seven-year trial, they absolutely couldn’t maintain the level they had in the DCCT after it ended.”
Kowalski projects energy and optimism. He is articulate, passionate, and determined, and his mind, like Brewer’s races a mile a minute. These traits would eventually lead him to become JDRF’s current CEO (Brewer served as JDRF CEO from mid-2010 to mid-2014). But back then, he was at the bottom of the JDRF scientific food chain and one of his first assignments was to attend a conference of the Diabetes Technology Society in Philadelphia. Prior to the main conference session, Kowalski was stunned to meet a group of scientists and patients working with a device that would continuously monitor blood sugars 24/7, a result that blood glucose meters could not match unless a person pricked their finger a few hundred times a day.
“Continuous glucose monitoring (CGM) was a holy grail,” Kowalski recalled of his encounter with the scientists. “I go to this meeting, and they have a CGM and they’re testing it. It alarms when your blood sugars are low or high, you see your blood sugar number all day long.’ It was the greatest news I’d ever heard. It was amazing.” And it offered the hope of achieving the tight controls recommended by DCCT and adopted by clinical organizations like the American Diabetes Association.
The next day, Kowalski attended the plenary session of the conference, and his mind was still buzzing with excitement about the CGM he had seen the day before. The first speaker was a renown T1D endocrinologist who declared that people with T1D can do just fine using the current tools of glucose meters, injections, and insulin pumps. When the Q&A began, someone in the room rose and said, “Doctor, I give you a lot of credit to stand up in front of a room like this and to say something so incredibly stupid and outrageous. That is the dumbest thing I’ve ever heard.”
The mystery man was Jeffrey Brewer and the notion that the available tools were adequate enraged him. Soon after the event, Brewer and Kowalski talked for the first time. Kowalski later said they were “peas in a pod” with a shared determination to improve management of T1D. Their objective was to persuade JDRF to commit research dollars to development of an artificial pancreas system (APS)4 – a system where a CGM would “talk” in real time to an insulin pump and direct it to dose exactly the right amount of insulin to safely control blood-glucose levels with limited intervention by the user. In short, the idea was to come as close as humanly possible to mimicking the function of the human pancreas by automating insulin delivery and relieving people with T1D from the burden of counting carbs, dosing insulin, and making life and death decisions based on gut and math 24/7.
[4] The term artificial pancreas has become common to describe the systems that were eventually developed. They are also referred to as Automated Insulin Delivery Systems. However, technically, the current devices do not mimic a real pancreas for several reasons, including the fact that the pancreas has other hormones involved in blood sugar regulation besides insulin, and they still require some user intervention. Regardless, in the period covered by this story, the term “artificial pancreas” was commonly used and thus that is the case here.
Chapter 3: The Patient Organization: Cure or Treat
An artificial pancreas was not a new idea. In fact, Dr. Ernst Freidrich Pfeiffer developed a machine called the Biostator in 1974, an insulin pump with intravenous (IV) continuous glucose monitoring and IV insulin infusion. The machine was the size of a refrigerator and was only feasible for hospital settings. Thirty years later, the idea of creating a wearable, user-friendly artificial pancreas remained mostly an idea, perhaps dabbled with by some scientists around the world but not of interest to diabetes technology companies or many others. In fact, a few years before the Brewer-Kowalski team formed, JDRF itself had set an organizational goal to develop an artificial pancreas. But it had not allocated any funds toward this goal mainly because senior staff and volunteer Board leadership believed passionately that diverting resources from curing T1D would break faith with donors and throw the organization off course from its singular mission and identity to cure T1D.
John Brady was a member of the JDRF Executive Committee at the time. His son Phillip had T1D. “The intensity and passion of those who had been involved for years was incredibly high. They did not see room for treatment in our mission. They argued we were founded to find a cure, not to treat the disease.”
Brady disagreed and quickly emerged as a key ally of Brewer and Kowalski. “What Jeffrey wanted made complete sense to me—to keep people alive and healthy until we find a cure. We were losing people, kids dying in bed overnight of low blood sugars. If we could automatically dose insulin and have everybody go to sleep and all wake up, that was an incredible victory.”
Brewer and Brady, along with a handful of other volunteers, worked to educate and persuade other key volunteer leaders. Kowalski was working the inside, trying to persuade his research staff superiors to go all-in on funding development of AP systems. He recalled that his bosses told him to stop ‘stirring things up.’ I said I didn’t care. We have got to do this.”
Early in 2005, and now a member of the JDRF International Board, Brewer “decided to force the issue of the artificial pancreas with the JDRF board in the following way: I made a directed gift of a million dollars that was contingent upon that money going to fund proof of concept research for integration of CGM and insulin pumps and development of algorithms for automated insulin dosing.”
Brewer remembered that “they said my gift will dilute the mission, and industry was already taking care of it. But they knew they didn’t want to turn away the money.”
Looking back at the Board meeting, Brady remembered the drama and tension. “It was not a slam dunk. It was probably the most emotional debate that I ever witnessed in all my time on the board. And it was far from unanimous. There was a significant portion of the board who thought it was wrong. This was not something that had strong widespread support, both as a project, nor as a redefinition of our mission to include treat.”
Kowalski recalled that some of the most influential Board members opposed accepting Brewer’s donation. “Some were vehemently against it. One of them was on the Board of Johnson & Johnson and he said ‘J&J’s research budget for diabetes is hundreds of millions of dollars. You’ll never make an impact.’ It was a huge throw down. And Rob German, the chairman, said, ‘this guy’s passionate, he’s willing to put his money up. Why don’t we get somebody on the board, set them up with Jeffrey and this Kowalski kid and give them six months to convince us you can spend a million bucks.’” Said Brewer: “They didn’t know partnering me with Aaron was basically putting the fox in the hen house.”
The study commission was led by highly respected Board member Charles Queenan. In October 2005, Queenan presented the study commission recommendations including “to pursue [an artificial pancreas] with the same intensity as other JDRF cure therapeutics goals.” According to Board minutes, the Board agreed to approve the recommendations.
With this, the Artificial Pancreas Project (APP) lifted off. It marked a turning point in JDRF’s research priorities as the organization fully embraced the commitment to keeping people healthy on the path toward a cure. Within a year, the emotional battles over cure vs treat would be largely forgotten. The JDRF commitment would help catalyze the most significant advance in T1D treatment in nearly a century. Between 2005 and 2024, JDRF would spend $171 million on artificial pancreas related research that would result in the commercialization of a series of systems that partially automate insulin delivery and dramatically reduce the daily burden of T1D while improving the daily and long-term health of people with the disease.
Chapter 4: “Now What Do We Do?”
There is a scene in the movie The Candidate where Robert Redford’s character, Bill McKay, has learned he was the upset winner for a U.S. Senate seat in California. Just before McKay goes down to speak to his supporters, he turns to his chief advisor and asks, “What do we do now?”
That was the exact question facing the small team at JDRF charged with creating an artificial pancreas. The diligence study had made one thing clear: this would not be your mother’s science project. It would have to run on multiple parallel tracks: the science and engineering track—developing algorithms—the mathematical formulas that would track data from the CGM and adjust insulin dosing minute by minute to keep blood sugars in a safe range; the regulatory track to understand what the FDA would require to grant approval of an artificial pancreas; the industry track to encourage investment and commercialization of AP systems; and the reimbursement track to persuade private and public health plans to cover the system upon approval.
It was unlike anything JDRF had ever undertaken. And it would need someone with a unique set of cross-sectional skills to lead. JDRF CEO Peter Van Etten had just the guy: Larry Soler, JDRF’s Washington, DC-based chief lobbyist who had organized and led a multi-faceted advocacy campaign to overturn bans on stem cell research and JDRF’s advocacy campaigns to ensure multiple renewals of the Special Diabetes Program (SDP) that provided NIH with hundreds of millions of dollars for T1D research since its original enactment in 1998. To Soler, his work was more than a job. He himself had been diagnosed with T1D in his early 20s.
And Soler had exactly the right partner in a recent hire who had unexpected bandwidth due to congressional paralysis on an unrelated issue. Her name was Cynthia Rice, a Harvard graduate with experience as a Senate staffer, working in nonprofits, and a stint staffing President Clinton’s Domestic Policy Council specializing in health policy. Soler and Rice were both preternaturally calm. Both were highly meticulous and organized, and both knew their way around the corridors of power in Washington, DC, which would be an epicenter of activity given the presence of the U.S. Food and Administration (FDA), the regulatory body that would have to approve for commercial use in the United States any artificial pancreas system. (Soler would leave JDRF in 2009 to become the first CEO of Michelle Obama’s anti-obesity nonprofit, Partnership for a Healthier America, and Rice would take over the lead role, seeing the project to its completion before she retired in 2023).
As the team organized and started building out a strategic plan in December 2005, the NIH hosted the first of what would become a series of key research and interagency conferences on artificial pancreas technology over the next decade. The focus was on exactly how to pursue the goal. It turned out that not everyone there felt the goal was attainable.
One of the attendees was a University of Virginia Ph.D. mathematician named Boris Kovatchev. Kovatchev’s father had been diagnosed with insulin-dependent diabetes at the age of 48 and it made a huge impression on him. “He had a couple of toes amputated. It was a really bad case.” Kovatchev especially grasped just how difficult it was for people with the disease to correctly dose insulin, and upon arrival at UVA in the 1990s, he quickly started specializing in T1D, including tinkering with algorithms to manage insulin dosing.
“There were debates at the NIH meeting whether it is even possible to do an artificial pancreas,” he recalled. “There was a prominent scientist who said it is not possible. There were essentially two camps, one was ‘let’s try it,’ and the other was ‘it’s not possible.’” Said Kowalski: “This prominent researcher stood up and said ‘you’re going to kill people.’” But despite some skeptical voices, Kowalski told the gathering that JDRF would move ahead and seek applications to fund key research to advance the project, especially in the area of developing the critical algorithms that would be the brains of the operation.
By August 2006, teams of researchers at multiple academic centers had received grants of about $300,000 each to take on various aspects of AP development. Some had dabbled in the field for years, others more recently. But collectively they were a Murderers’ Row of T1D scientists. Bruce Buckingham at Stanford, Bill Tamborlane and Stu Weinzimer at Yale, Kovatchev at UVA, Roman Hovorka at the Wellcome Trust-MRC Institute of Metabolic Science at the University of Cambridge in the UK, and Frank Doyle, Howard Zisser, and Eyal Dassau at University of California at Santa Barbara (UCSB)5 were among the grant recipients.
Another early grantee was the Jaeb Center for Health Research, founded by Roy Beck. His organization’s role wasn’t the headline-grabbing work of conducting high-profile trials, but it was a critical component of the project. For the next decade, Jaeb coordinated virtually every important clinical trial, engaged with the FDA on trial design, captured and analyzed data from the trial, and supported the publication of the research in peer-reviewed scientific journals. It also served as secretariat for the JDRF-created Artificial Pancreas Consortium, a series of meetings over the years bringing virtually every major researcher, funder, and company in the field together to exchange information and ideas. “What Jaeb did was herd some of the wildest, cockiest people,” said Kowalski. “They helped us enforce the rules of the consortium so people weren’t going off on their own screwing things up. Roy is probably one of the most important people in diabetes that nobody’s ever heard of.”
While researchers were energized, two immediate challenges emerged.
One was the U.S. Food and Drug Administration (FDA), the regulatory agency that reviews and must approve for commercial use the safety and efficacy of all medical devices and drugs. In a meeting in the early months of the project, JDRF’s team met with FDA officials to explore what data and studies the agency would want to see as a basis for reviewing AP systems. The response was discouraging.
“We went into their conference room and, boy, did they shut us down,” recalled Soler. “They basically told us that if we are thinking about testing these systems in humans we should instead be talking about testing in mice. I remember leaving and feeling, whoa, this went really bad.”6
The second concern was the reliability of what would be the nerve center of the AP system—the continuous glucose monitors that Kowalski was so enthused about months earlier. FDA had approved early versions of CGMs in the late 1990s, but they did not actually perform real-time blood-sugar monitoring. In 2006, it approved two newer “real-time” systems developed by the major device maker Medtronic, and a new start-up named Dexcom. But the FDA only allowed these CGMs to be used as a secondary measure of blood-glucose checking because it still felt that the finger prick meters were more reliable.
“FDA didn’t allow the use of CGM data for decision making and could not reconcile that insulin would be dosed automatically based on a reading from a device they deemed inaccurate,” said Sanjoy Dutta, currently chief scientific officer and head of Research at Breakthrough T1D (formerly JDRF) who joined the JDRF APP team a few years after the project started. “The initial CGMs had high levels of inaccuracy,” said Dutta. “They were the Achilles Heel of developing AP systems.”
FDA’s concerns about CGMs were troubling enough without the fact that health insurers declined to cover them (they could cost up to $5,000) because they were not persuaded that they were more accurate than existing glucose meters, a view reinforced by the FDA’s decision to approve them only as secondary measures. If insurers would not cover CGMs, it was even less likely they would cover even more advanced and complicated AP systems, and without some certainty around coverage the prospect for AP development would be dim.
[5] Each of these researchers were part of larger teams that participated in the groundbreaking work, but it is impossible to list all the names here.
[6] Typically, research includes a preclinical phase where drugs and devices are tested in animals, often mice. But these studies are costly and lengthy and would set the AP project back years before it got off the ground.
Chapter 5: The Big Gamble
“We realized that if the sensors weren’t successful, we couldn’t get to a closed loop,” said Kowalski. “And nobody believed in the sensors. They all sucked. And we went around and asked ourselves what we need to do” to prove that even less precise early sensors would still be better for patients than glucose meters. The discussions led JDRF to take a big gamble and invest $10 million to conduct a yearlong, multi-center trial of the new sensors now being developed by Medtronic, Dexcom, and a third player, Abbott, to prove to insurers that CGM were more accurate than glucose meters.
“We made all the decisions about this trial, but we consulted with the companies,” Rice said about the team’s meetings and calls with device makers and health insurers. “The companies were very risk averse.” But ultimately, working with Jaeb, and believing they could design a sound study that would prove the latest CGMs were superior to any existing blood meters, JDRF moved forward. The trial that would be historic for its folly or brilliance launched in September 2006.
Kowalski remembers a meeting with Claudia Graham, a senior executive at Medtronic. “She said to me ‘you guys could screw up this whole field if the trial fails; this whole field will be dead.’” Graham remembers the conversation. Her comment was rooted in her concern that the first generation CGMs were “kludgy” and finicky and hard to use for many. Concern about doing the CGM study) was that these were all first-generation devices. “I was very worried the results would reflect those imperfections at the time.”
JDRF’s Soler said: “The trial was a huge gamble. It was hugely expensive, and the goal was to show that people who use CGMs do better than people who don’t.”
If that goal was not achieved, Graham’s fear would be reality.
Chapter 6: The Device Makers
Al Mann was the son of a Portland, OR grocer and a mother who was a pianist. Mann’s brother was a violinist who became a founder of the famed Juilliard School of Music, and his sister was a concert pianist. But Al went another direction, getting a degree in physics and becoming a serial entrepreneur, starting companies in fields ranging from aerospace to cardiac pacemakers. Eventually, in the late 1970s, he turned to diabetes, starting a company called MiniMed which developed insulin pumps. In 2001, he sold MiniMed for $3.7 billion to one of the country’s leading medical device makers, Medtronic. By 2005, Minneapolis-based Medtronic was the undisputed giant in the T1D device space. Al Mann remained involved, and he was the person who flatly stated that at the 2005 NIH meeting that AP systems would “kill” people.
To JDRF, engaging industry was a critical objective because products are not developed and sold by academic scientists working in labs. Indeed, a foundational tenet of the AP Project was to catalyze industry involvement. In fact, in one of Brewer’s presentations to the JDRF Board, he had a slide which simply said: “Without JDRF, still ten years away.” So, JDRF made it a priority to attract Medtronic to AP development. But the company was not taking the bait.
“They were sitting fat and happy owning the insulin pump business,” said Brewer. “We went out to their headquarters in Minnesota for a big dog and pony show. I remember they had an iceberg slide which showed nothing at the top, but they told us what’s going on with AP was confidential and below the surface and that we didn’t have to worry about it, and we should go back to funding the cure. But we knew they in fact they had few people working on it under a small NIH grant and they weren’t putting a dollar of their own capital into the AP. They didn’t see the need to do so. It wasn’t going to help them expand the pump market which they already owned.”
“The problem with Medtronic was there was a certain degree of arrogance,” said Graham who retired from Medtronic in 2008 (she eventually joined Dexcom. “They thought it was all about the pump. They had hundreds of thousands of pumps in the field with four-year warranties so for them to easily shift over [to AP systems] was a big business hurdle.”
Medtronic’s seeming slow pace created a new imperative. Abbott, Dexcom, and Johnson & Johnson’s diabetes subsidiary Animas were much smaller players and fighting to gain a foothold in the CGM market. Leaping directly to AP development was seen as high risk.
“For JDRF the fundamental problem we had was to create competition where there was no meaningful competition in the pump market,” recalled Brady. “Medtronic was the gorilla and had the ability of doing it, the technology, and the resources but there was no competitive market incentive for them to move forward.”
Chapter 7: The Inventor
When Dean Kamen was in his teens growing up in Long Island, NY in the mid 1960s he started making and selling to local bands homemade lighting control systems. Around this time, his older brother Barton had earned a Ph.D. in pharmacology and was focused on pediatric oncology.
“My brother would come home and explain what he was doing in neonatal ICU. He had some ideas on how to do cancer treatments better in babies. He wanted something that could give them very small amounts of drugs and to time the dose in intervals instead of running on an IV drip.” Kamen developed a pump device that met his brother’s specifications, using off-the-shelf components including the base of a standard butter dish. He made the units in his parent’s basement and drove them to his brother at Yale. “It wasn’t a business,” he recalled. “I was just trying to help my brother save babies.”
But the brotherly collaboration would change diabetes history. A few years later, Dean Kamen was at Worcester Polytechnic Institute. His brother Barton showed the pump device to a colleague at Yale. The colleague was the same Bill Tamborlane, who would co-lead some of the most seminal AP trials years later. But at the time, he was working with pregnant women with T1D.
According to Kamen, Tamborlane envisioned adapting the pump system to dose insulin. He imagined a pump that would provide a steady baseline, or basal, dose of insulin through the day, and allow the user to give a higher, or bolus, dose at mealtimes to account for the carbohydrates consumed. Kamen took on this challenge and developed a wearable pump weighing 17 oz. It was the first insulin pump in history. It was known as the “blue brick” because this time Kamen could only find blue butter dishes. Soon, Kamen created his first company, Auto Syringe, with the blue brick at its center. In 1981, the drug company Baxter International purchased Auto Syringe.

After the sale to Baxter, Kamen continued to create inventions in a range of fields, including healthcare. But he stayed abreast of the insulin delivery and CGM market, and, by 2005, his mind returned to whether he might invent a way to automate insulin delivery combining a CGM with an insulin pump. “I thought if they can make sensors that can measure blood glucose levels every couple of minutes we ought to be able to do a closed loop control so I said, ‘I got to go back and develop a technology that eliminates the syringe and its mechanical limitations, I got to make it small and light, and I’ve got to invent a measuring system that can measure down to fractions of a microliter how much insulin I am delivering every time.’”
So, it made sense that when JDRF began to explore options to develop AP systems, Kamen, by then founder and CEO of DEKA Research and Development Corporation and now world famous for his invention of the Segway, would be one of their first stops.
It soon became clear that the dream of creating competition might be realized.
“We went and toured the engineering facility, and we ended up making multiple visits up there,’ said Soler. And in the process, they learned that Kamen had been exploring with Abbott the possibility of creating an AP system pairing Abbott’s Navigator CGM, with Kamen’s new pump and dosing algorithm that his team developed. Soler recalled the team’s emotions as the dialogue continued. “You had a company that had a very promising CGM. They were building a significant insulin pump with Dean. Dean was an innovator. Dean had built the first insulin pump and has been involved for a long time and had a record of innovation. And so, we got really charged up by it, and we went for it.” “It” in this case was putting together a multi-party deal between Kamen’s DEKA R&D, Abbott, and JDRF.
“We had negotiations about the different IP rights, how much funding we would invest based on milestones, and what our royalty rates would be,” Soler recalled. “It was a long process, but by 2008 we got to the point where we had a deal that was agreed to by the parties.” As the deal wrapped up, Abbott announced that the President of its diabetes division who had been its lead negotiator would move over to become president of the cardiac division. The news did not trigger any alarm bells at JDRF.
“We scheduled an in-person meeting at the JDRF headquarters in New York to sign the final documents,” Soler said picking up the story. “It was in the evening and lower Manhattan was dark. The lights were out in every office except for the one conference room. We had the papers all laid out for signature and were waiting for the new executive in charge, Heather Mason, to arrive. But we kept getting alerted that the company plane was delayed. And finally, she contacted us directly and said she didn’t want to do the deal. So, she never showed up. We thought we would be celebrating. But she left us waiting.” The deal was dead.
Chapter 8: The Tide Turns
Despite the crushing disappointment over the Abbott deal, there was still cause for hope. For starters, the scientists JDRF funded in 2006 were delivering promising results.
In 2007, Doyle’s team at UCSB had published data showing that they had developed a closed loop system using a Dexcom CGM and an Omnipod insulin pump made by Insulet that communicated with one another using an algorithm that adjusted the insulin infusion rate every five minutes based on predicting future glucose levels. It was an important landmark in AP development.
At UVA, and across the Atlantic in Padua, Italy, Kovatchev and Claudio Cobelli, were collaborating to develop a simulator—a computer model—representing 300 different metabolic systems that allowed them to test scores of real-life scenarios, such as creating scores of different types of meals with varying carbs, different forms of exercise for different periods, and then testing multiple different algorithms to see, among other things, how much time the simulated “people” were in range, what risks emerged, and how many hypoglycemic events occurred. The idea was to prove to the FDA that these simulated models were an accurate substitute for testing artificial pancreas algorithms in humans not animals.
In January 2008 the FDA accepted the simulator as a substitute for animal testing. Three months later, Kovatchev’s team started an in-hospital human trial at UVA. Cobelli and a third colleague, Eric Renard in France, quickly followed suit. “The simulator saved at least five years of animal studies because we didn’t require an algorithm to be tested in an animal model to be deemed safe and effective before going into human studies,” said JDRF’s Dutta. “That entire chunk was eliminated thanks to Boris and Claudio.”

Also, in 2008, Weinzimer and Tamborlane at Yale published results of a first in human study of 17 adolescents using a rudimentary but nonetheless fully automated closed loop system. They reported that “closed loop glucose control using an external sensor and insulin pump provides a means to achieve near-normal glucose control in youth with T1D during the overnight period.” When the team presented their findings at a JDRF research summit, “people were literally crying,” Weinzimer recalled.
In even bigger news, in September 2008, JDRF’s big gamble to run a CGM trial paid off. The trial proved conclusively that CGMs are associated with improved blood-glucose control in adults with T1D compared to those who used standard therapy. “People still cite it as the landmark JDRF CGM study,” Kowalski said proudly. “I remember when that paper was published in the New England Journal of Medicine that it was like the whole world changed.”
The goal of the paper had been to persuade health insurers to initiate coverage of CGMs. But even before it came out storm clouds formed. One of the largest insurers, United Healthcare, was on the verge of issuing a non-CGM coverage decision. It would have sent a potentially fatal message to other payers, potentially crushing the CGM market before it was off the ground, and even taking down the artificial pancreas market still in its infancy. JDRF tried to reach out to its contacts at the company but hit a brick wall. Cue its network of well-connected volunteers.
“We learned that United Healthcare was planning a CGM non-coverage decision,” said Rice. “I put out an all-points bulletin to our network, asking if anyone had connections to senior executives at the company. Lo and behold, one of our leaders, Pam Sagan, had been friends for many years with a UHC executive and his wife, and that executive had since become CEO of the company. Pam was able to get through to him quickly and share that important new research was going to be published shortly, and it would be in UHC’s best interest to delay its non-coverage decision to reconsider the new evidence,” Rice recalled.
“The CEO got the decision delayed and a meeting for us to brief senior medical staff on the new findings. United Healthcare—the largest health plan in the country—ended up covering CGMs based on the JDRF study results and this volunteer intervention.” Soon, virtually every insurer would announce a favorable coverage decision.
But there were still some headwinds. Kowalski kept hearing grumbling that the project was going nowhere because there was no obvious commercial product. Sure, the lab research with somewhat jerry-rigged systems was nice but they were too cumbersome and complicated to actually be developed into a viable, wearable commercial device.
Kowalski knew they had a point. Industry needed line of sight into where all this would lead not happy talk and assurances from JDRF and scientists. What emerged would eventually be known simply as The Roadmap, a set of six boxes with iterative devices that suggested concrete products with escalating complexity and automation.
“When we got the CGM trial data, apart from the fact that the sensors helped people, a big ‘ah ha’ was nobody appreciated how much time people were spending with high and low blood sugars every day,” said Kowalski. In fact, the data found that if you were hitting one of the targets recommended by clinical organizations based on the DCCT data, “you were still spending almost 12 hours a day with a high blood sugar and 80 minutes with a dangerously low one. It blew people’s minds.”
This data crystalized a path forward. “I had the idea that a first product could turn off the pump when people are low” so that it didn’t continue to dose insulin and plunge them into severe hypoglycemia. The so-called low glucose suspend system would not be the ultimate goal for automated insulin delivery, but it would be less daunting to develop. And by suspending glucose when the CGM reached a specific low reading it would help overcome FDA’s concerns about rogue glucose sensors triggering a dangerous low blood-sugar event by continuing to dose insulin well past the safe level.
In the end, Kowalski created six product visions grouped into boxes. They culminated in Box Four, a “hybrid” closed loop where insulin delivery would be mostly automated except at mealtimes when the user would have to “bolus” to account for the carbs consumed (thus hybrid closed loop, not fully closed loop), Box Five where, a fully automated closed loop, and Box Six, a system that not only dosed insulin but other pancreatic hormones that impact glucose control. Published in Diabetes Technology and Therapeutics in September 2009, the paper for the first time laid out a manageable development plan for companies that made the ultimate goal seem more attainable. Looking back, Roy Beck said that Kowalski’s seminal paper “has been the roadmap for the last 15 years in artificial pancreas development and is still cited.”
And in January 2010 JDRF’s efforts to engage industry paid off. It struck a deal with Johnson & Johnson subsidiary Animas to build an AP closed loop system using a CGM provided by Dexcom. JDRF committed $8 million over three years to support the project. Recalling how important this was, Soler said: “We had a major medical device company make a public declaration that they were going to create an automated insulin delivery system, and it really galvanized the attention of the diabetes industry.” (The irony was not lost on some that in the JDRF debate whether to accept Brewer’s funding, one leading opponent had declared that J&J was never going to invest in the artificial pancreas space. Five years later, they did just that.)
Chapter 9: The Allies
David Panzirer’s daughter Morgan was diagnosed with T1D in March 2007. Immediately, his grandmother, New York real estate mogul Leona Helmsley, made a $5 million donation to JDRF and another organization in the T1D field. Upon her death five months later, her will stipulated that her assets be liquidated and transferred to a charitable trust, Helmsley Charitable Trust (HCT). Panzirer became a trustee, and together with his co-trustees they set up a series of core programs including a focus on T1D. If Morgan’s diagnosis wasn’t enough motivation, his other daughter Caroline was diagnosed in 2017. Over the years, Panzirer has developed a visible, respected, and well-cultivated image as someone willing to be outspoken and willing to disrupt the status quo if he feels it would help the lives of his kids and the entire T1D community. HCT and JDRF have partnered and collaborated on numerous transformational initiatives, with occasional friendly disagreements.
HCT would prove to be a powerful ally, lending its voice to the effort to educate the FDA on T1D and tear down its regulatory barriers. One of its major contributions to that effort was to build the first-of-its-kind registry on T1D patients. It started in 2007. “We kept on asking when we started where the data was to show how people in the U.S. are doing with T1D,” Panzirer recalled. “The data did not exist. It was mostly thought of as a safe and managed disease.” About three years later, during JDRF’s campaign to turn the FDA around (described in the next chapter), Panzirer presented the registry data to the agency. “The registry collected data on 3,000 people in 27 clinics and showed that T1D was neither managed nor safe,” said Panzirer. “I presented the data myself and their chins were on the floor.” Said Kowalski, “The registry was very helpful. It was super important to help show the unmet need.”
HCT also stepped in to beef up JDRF’s team. In 2010, with the work expanding and the possibilities for success growing, HCT provided a transformational grant providing resources for JDRF to hire an expanded team for the AP campaign, including Campbell Hutton, a regulatory expert, scientist Sanjoy Dutta, Linda Johnson, an expert in managing alliances with industry, and Marlon Pragnell, another research scientist. “It was huge,” said Soler. “They became central members of the team.” “HCT provided resources that allowed us to take the JDRF Artificial Pancreas project to the next level,” Kowalski said.
HCT would also become a leading funder of the multi-hormonal “bionic pancreas” that Boston University researcher and T1D parent Ed Damiano was developing. The Kowalski roadmap had included such a system as the final Box Six, the ultimate manifestation of a real pancreas. But JDRF’s research priorities focused in these years around systems it felt were more realistic from a developmental and business standpoint, and HCT filled the gap by investing in Damiano’s project.
In 2012, Panzirer shook things up a bit more. With concerns about CGM sensors continuing, Panzirer approached JDRF with a proposition. “I went to them and said that the sensors are not good enough and that Helmsley is going to put up $12.5 million and I want you guys to match it, and we will do an RFP to companies who can improve sensors,” Panzirer said. Kowalski remembers that initially the idea of potentially providing direct funding to companies like Medtronic did not go over well at JDRF. Kowalski said the idea that JDRF would collaborate with the largest medical device maker in the U.S.” was very controversial. “But in the end, the two combined to provide $17 million to Medtronic to improve sensor technology. Notably, the deals contained royalty rights if the grants resulted in actual commercial products. While those sensor investments did not immediately bear fruit, they would ultimately result in meaningful improvements in Medtronic sensors that are currently coming to market. “To date,” says Panzirer, “we’ve gotten $42 million in royalties back.”
The other force multiplier was NIDDK. Prior to the launch of the AP Project, NIH had for years been providing small grants to researchers dabbling in AP research. But as JDRF stepped up its campaign, NIDDK would join it, leveraging funds from the Special Diabetes Program that JDRF helped create and sustain. Four years into the project, in 2009, it disbursed $23 million in research grants that added major accelerant to the project, and continued NIH funding eventually led directly to algorithms that are being used today in several commercial AP systems.
Chapter 10: The Regulators
Five years into the project, there was cause for optimism. But the FDA remained a major problem, even an obstacle.
From the start of the AP Project in 2005, there was frustration with the position of the FDA towards the technology and its potential. By 2010, despite the approval of Kovatchev’s simulators to replace mice trials, these frustrations were boiling over as FDA was continuing to raise barriers to conducting clinical trials, and generally showing little interest in advancing the technology. In 2010, Brewer became CEO of JDRF. “The FDA had been very unhelpful. There were people there overseeing the field who didn’t think there was any need for technology to manage insulin and glucose. They felt there was already an acceptable treatment and people should do what their doctor directed, take insulin as prescribed, and they would be fine,” recalled Brewer.
JDRF and researchers were pressing FDA to allow outpatient trials not just ones in highly supervised hospital settings. And it wanted the agency to develop an Artificial Pancreas Guidance, essentially a roadmap laying out the steps that needed to be followed to get device approval: what trials are required, how they should be designed and what their endpoints should be, how many patients had to be included and what ages, what technology might be used to capture data like blood-glucose levels, among other key regulatory issues.
“There were multiple years of FDA wanting more and more data before trials could progress, which we saw as over-regulation,” recalled Kowalski. “So, frustration boiled and boiled and boiled, and we finally went to Jeff Shuren who was the new head of the Center for Devices and Radiological Health (CDRH) at the FDA and asked him to get involved.”
But they weren’t getting anywhere, so in July, JDRF brought together a broad set of stakeholders to develop recommendations on how to conduct outpatient trials safely. “In November we presented the recommendations to the FDA and other experts at an FDA/NIH public workshop,” Rice said.
It did not go well, and JDRF decided to turn up the heat.
“We were so concerned with the dismissive reaction from the lead FDA reviewer at the workshop that the same day, right after the workshop ended, we went to Larry Soler’s house nearby to confer about next steps—including me, Aaron, Jeffrey, Larry, Campbell Hutton (the in-house JDRF regulatory expert), and our regulatory consultant Phil Phillips,” recalled Cynthia Rice. “At that meeting, we decided we would draft an actual guidance document, in the format that the FDA could release, based the earlier recommendations.” This would lay out a pathway to get to the “Holy Grail” of Box Six in the Kowalski roadmap. The guidance document was submitted to the FDA in March 2011.
Over the next six months, JDRF waged an intensive, multifront advocacy campaign to send a message to FDA that it was time to move faster and more constructively on AP systems. It started in March when volunteers from all over the country flew into Washington, DC for JDRF’s annual Government Day and swept across Capitol Hill asking lawmakers to sign a letter being circulated by the House and Senate Diabetes Caucus Co-Chairs7 to the FDA to promptly issue a guidance that reflects the view of expert clinicians and researchers.
In June, JDRF staged its biennial Children’s Congress to educate lawmakers about T1D. Long-time JDRF ally Senator Susan Collins (R-ME), Chairman of the Senate Aging Committee, agreed to schedule a hearing to press FDA officials about the lack of progress in advancing artificial pancreas systems to patients. The stagecraft would be designed to create an emotional exclamation point on the issue as the 200 delegates between the ages of 5 and 17 sat in chairs and on the floor in front of the dais in the hearing room.
FDA was getting the message and tried to get ahead of things. It released a guidance document the very same day of the hearing. The bad news is the guidance was narrow—it only covered a pathway for a low glucose suspend version of the AP—Kowalski’s Box One, a first-generation device already available and in use in more than 40 countries around the world that researchers already felt they could leap beyond. Not only was it viewed as too conservative, but there was also another problem: said Rice: “It was terrible.”
In September, joined by key clinician groups like ADA, individual researchers and clinicians, JDRF submitted comments blasting the FDA’s June draft. In October 2011, it launched an online petition calling on FDA to issue a pathway to accelerate AP development. In short order, over 120,000 people signed the petition, most with a direct connection to T1D. And one month later, Senators Collins and Shaheen held a press conference, joined by an 11-year-old with T1D, Caitlin Ryan, urging the FDA to issue clear and reasonable guidance for AP that avoids unnecessary delays. A foot-high stack containing all the names of the petition signers was a prominent prop.
The final salvo came that same day. Full-page ads appeared in The New York Times and Washington Post headlined: “The FDA can help save the lives of those with type 1 diabetes.” Pictured on the top of the ad was an adorable young girl named Piper, an eight-year-old who had been diagnosed at age 3. The ad copy said: “Piper has type 1 diabetes. One in twenty people like Piper will die from low blood sugar. In fact, kids and adults are dying every day from low blood sugars or complications caused by type 1 diabetes. In the next few weeks, FDA has a chance to show it is leading the world in medical innovation not standing in its way. It will lay out the pathway to bring to market the first artificial pancreas, a lifesaving technology now under development. And the most revolutionary treatment in diabetes since the discovery of insulin. Three million kids, teens, and adults with type 1 diabetes are counting on the FDA to get it right. Our lives and health are at stake.”
To this day, people at FDA at the time, JDRF staff, and volunteers, remember “the ad.” It remains the single most public and aggressive action the organization has ever taken to advance research to treat and cure T1D.
In December, FDA released a second draft guidance on outpatient testing of AP systems. But it too didn’t meet the expectations of the T1D community. Once again, JDRF made clear to FDA that it was still falling short.
Shuren, who plans to leave the FDA by the end of 2024, remembers this period well. “JDRF asked me to meet with them and they laid out their concerns,” he recalled. “In particular, they talked about problems with the office and the FDA team. So, I started having a series of meetings with the team, JDRF, and others; and it became pretty clear to me, although they were well-intentioned, we were not engaging in a constructive manner.”
Shuren got the message, and he made his move. He decided to pull oversight of the entire AP project from the original group. “I determined that it made more sense to move the AP technology [and insulin pumps] to another part of the center that dealt with other diabetes related technology like glucose meters. So, I put all diabetes related technology in one place.”
Stayce Beck, a bioengineer, was the lead member of the new team. “The ads changed the trajectory of my life,” she said years later. “It spurred FDA to take the AP and give it to my group and they asked me to lead the project.” Early in 2012, the new team took the JDRF draft guidance “to heavily resource our version” and “to finalize it in a way that aligned with what they were thinking.” It took another 10 months but finally, in November 2012, FDA issued a new and improved guidance.
Finally, the AP community had leaders at the FDA who made clear they were there not to obstruct but to facilitate while staying true to the agency’s highest standard of ensuring any approved system was deemed safe and effective.
Said Shuren: “I do give JDRF credit for pushing, for saying there’s a real need for this, and raising the clarion bell on concerns with the center.”
“The companies would always tell us that FDA would never allow this to happen,” said Kowalski. “But now they had a guidance document from FDA saying it can happen if you do these trials this way, and the trials were the right ones, so the guidance was a seminal moment. It was huge.”
“The guidance opened the floodgates,” said Dutta. “Now companies knew how to do trials, who to recruit, how long to do them for, what do they need to prove. Now people knew there was a path as opposed to driving in the dark.”
Indeed, the research community did move into overdrive. Outpatient trials were launched and completed in waves. Kovatchev, Cobelli , Buckingham, Hovorka, a team at Mt. Sinai in New York and the Mayo Clinic in Minnesota, Ed Damiano at Boston University, all conducted trials of patients in different settings with different algorithms and system components between 2012 and 2016. They started with overnight outpatient trials, moved on to multiday in-home trials, and then to three-month and then six-month “real-life” trials. There were trials in diabetes camps and trials at ski camps. The pace was dizzying but the results were exhilarating. Time and again, the trials demonstrated both the safety and efficacy of automated insulin systems.
[10] Senators Susan Collins (R-ME) and Jeanne Shaheen (D-NH) chaired the Senate Diabetes Caucus and Representatives Diana DeGette (D-CO), and Ed Whitfield (R-KY) chaired the Congressional Diabetes Caucus.
Chapter 11: The Hackers
Progress was happening. But for many with T1D it was agonizingly slow. Most could only wait. Some decided they were tired of waiting.
Bryan Mazlish had founded an automated stock trading business where he and his team wrote algorithms to buy and sell stocks. His wife Sarah had been diagnosed with T1D when she was 12. In 2011, their middle child, Sam, was diagnosed with T1D at age 5.
“I am embarrassed to say how little I understood what my wife was going through on a daily basis until I was helping my son,” said Mazlish. “I never really understood the pernicious impact that the disease has on someone. You’re essentially driving a car on a curvy mountain road 24 hours a day, seven days a week with no time off even when you’re sleeping.”
Sam started wearing a CGM, but the couple was frustrated with the technology, largely because it was hard to hear the system’s alarm go off at night signaling a problem. “We wanted to be able to hear our son’s alarms at night and also monitor his glucose,” said Mazlish. Working with his father-in-law and brother-in-law, both engineers, they managed to figure out a way to remotely monitor Sam’s glucose levels and ensure they would hear the alarm when Sam was in trouble.
Excited about this step forward to make their son’s life safer, Mazlish asked his wife what else they could do, “and she said she had just had a rough night and if she could just wake up with a perfect blood sugar every morning that would be amazing.” The lightbulb went off.
Sarah and Bryan designed a do-it-yourself (DIY), or hacked, prototype artificial pancreas using an android phone and an algorithm they created to communicate with the pump and CGM. Sarah was the beta tester and wore the system the first night after the pair felt they had debugged it. “The first night she wore it, she slept one of the best nights of her life, but I was basically up all night monitoring the machine, making sure nothing bad happened,” said Bryan. Sarah used it for a few more months, they continued to tweak it, and when the couple were completely comfortable in it, they put it on Sam.
One other person was excited about the system. Aaron Kowalski. “Bryan calls me one day and says he wants to show me what he did. So, he comes to 26 Broadway (JDRF’s headquarters) and shows me his cell phone and it has his son’s glucose reading on the screen. I was like, ‘what the hell, what is this?’ And he says, ‘Oh, it was really easy. I just hacked it.”
Word started getting around about the system in T1D circles and Mazlish tried to get companies interested. “I talked to all the existing device makers, CGM companies, pump companies, and showed them what we had done, the data from Sam and Sarah and how amazing it was,” said Mazlish. “I said I would come work for them. But there were a lot of questions about whether FDA would approve it and a lot of hesitation and not a lot of urgency.”
Indeed, FDA was direct. “We were absolutely unenthusiastic,” said FDA’s Stayce Beck. “We did not want people to resort to these systems. We wanted to give them approved options.” If it was made available to others, even at no cost, the device would require FDA approval. In the end, the agency sent Mazlish a message: if you don’t try to promote this or make it widely available, we won’t act against you.
But by then others had taken up the DIY mantle.
Dana Lewis was diagnosed with T1D when she was a 14-year-old girl in high school in Huntsville, AL. As an adult she moved to Seattle in 2010 where she worked for a nonprofit hospital system helping how it and its doctors could effectively use social media as a communications tool. In 2013, she met Scott Leibrand, a network architect who would become her husband. Frustrated with existing diabetes management tools, they began to tinker with existing systems and in the summer of 2014, they met Ben West, another hacker who joined their work.
“By late November we had closed the loop,” said Lewis. They had in effect created a hacked AP system that predicted and prevented high and low blood sugars. In short order, the team built a website to tell the world about the OpenAPS, an open-source system that would allow others to replicate their creation. In this case, the FDA was powerless. “We weren’t distributing anything but ideas,” said Lewis. “That’s not against FDA rules and we felt pretty comfortable with open source and sharing it.” A few years later, a programmer, Pete Schwamb, took on the challenge of developing a hack that would allow users of the popular Insulet Omnipod pump to also adopt a hacked AP system.
Brewer, who would eventually leave JDRF to start Bigfoot Biomedical, with Mazlish and others believe the DIY movement played an important role in motivating the FDA to move faster. “DIY drove FDA crazy,” said Brewer. “It caused the FDA to put pressure on companies to move faster, they wanted it to go away and so they became advocates for companies to step up their game and move quickly to get away from what the FDA thought was the ‘crazy’ stuff people were doing themselves.”
Chapter 12: The Patients
Starting in 2008, hundreds of kids and adults with T1D around the world volunteered for clinical trials to test the emerging AP systems. Early on, the risks were real, and it took a lot of trust for people to rely on new-fangled technology to keep them safe. But they did it anyway.
Tom Brobson was diagnosed with T1D in July 2004 at the age of 44. “I was not the typical adolescent with T1D, and I was just trying to cope and remap my life. Then I saw Mary Tyler Moore8 being interviewed by Larry King on CNN and I donated to JDRF.” One year later, while working at Virginia Tech as a development official, he heard that JDRF was seeking to hire a major gifts officer, so he applied for the job, and was hired. Brobson would soon become one of the most visible and inspiring JDRF figures when he became one of the first persons in the world to wear a closed loop AP system as a participant in Boris Kovatchev’s hospital trial in 2008.

“They have two laptops set up,” he recalled of the experience. “I have two sensors on me. And I have an insulin pump. The two sensors were in case one failed. It was crazy just to walk down the hall to the bathroom because they had to put everything on big metal cart and wheel me down with all the wires connected. I ate dinner and watched the algorithm do a better job than I had done ten days prior. Most of all, that night, the big difference was I slept. When I ran things, I bounced low like six times through the night but when the system took over and ran things I avoided all that. And I remember sitting there texting Aaron and saying, ‘Oh my God, this thing works.’ It was a powerful moment.”
Brobson was also in the first UVA outpatient trial where the technology had transitioned to a portable system with a smart phone linked wirelessly to a glucose sensor and insulin pump. “We’re walking around with these phones with a stoplight design, a red, green, and yellow light with green indicating you were in a safe range, yellow that you were moving in the wrong direction, and red meaning that you were having a low or high blood sugar,” recalled Brobson.
Joshua Davis was diagnosed with T1D in 2009 when he was 11 months old. Joshua’s dad Brian had been diagnosed with T1D in 2004 right before his tenth high school reunion, so the family had some familiarity with the disease. But dealing with an infant was a whole different challenge.
“It was heartbreaking and just completely devastating,” said Shannon Davis, Joshua’s mom. “I went from learning how to carb count breast milk and baby food at diagnosis, because at the time that’s all he was eating, then carb count goldfish cookies when he was a toddler, figuring out how many goldfish did he throw on the floor. You see other moms pushing their babies in a stroller and they’re just kind of handing ’em food while you’ve got to stop and count each tiny goldfish that you’re giving your child. And then the sleep, we just didn’t sleep. Typically, we would check his blood sugar before he went to bed, and then we check it again at about 10 pm, then about 1 am, and then about 3 am, and then we would try to get until 7 am. Joshua actually learned how to eat a banana in his sleep to elevate his blood sugars.”
At age 5, Joshua had the opportunity to participate in a UVA outpatient trial. His parents said the choice was his. “I was really excited because I was already doing sports so the idea of being able to run around without having to worry about going low or having to stop and eat a snack in the middle of playing was a freeing feeling,” he recalled.

Joshua and Shannon headed to the Wintergreen Resort in Virginia where they would room together while Joshua wore the AP system. For several years Joshua had been on a CGM and a pump, so Shannon was used to responding to the alerts and helping her son make safe decisions on dosing insulin. But the camp was a big change.
“I can vividly remember the first night of the trial,” said Shannon. “The doctors said we needed to trust that they are watching our kids on a computer, and they told us we were not to respond the way we normally would so the system can do what it needs to. And I remember when Joshua woke up that first morning, he said he felt so good and I said, ‘Baby, you slept the whole night.’”
Alecia Wesner was diagnosed with T1D in 1979, and her parents became active with what was then called JDF. Growing up, she had two dangerous lows where she fell into unconsciousness. Eventually, she wound up in New York City in 1998. Soon, though, she was diagnosed with diabetic retinopathy, one of the common complications of T1D that can lead to blindness, especially if a person does not maintain excellent blood-sugar control and seek available treatments. As the AP Project took root, Alecia joined the local JDRF chapter. “At one of our Board meetings, Tom Brobson spoke, and he talked about being in trials at UVA, and I said I need to do that.”
She wrote to the UVA team, and they told her there was an upcoming trial that she qualified for at UVA. Months went by and then she was notified that they were no longer needed for that trial because it was no longer necessary as the technology had moved forward. But the email concluded by revealing that the next phase of AP trials were moving to other test locations including Mt. Sinai Hospital in Manhattan and she should contact the lead principal investigator at Mt. Sinai, Dr. Carol Levy. “When I got the email, I was sitting in her office.” Carol Levy was Alecia’s endocrinologist. Alecia got into the trial.

“It was amazing. I went to work every day. Nothing in my life was different. And then after work I would rush back to the hotel and they would set everything up and once we were in bed for the night, we got to turn the unit on. And they observed us while we slept. I knew from my own experience that I had been having problems going low at night then bouncing back up in the morning and sleeping through the alarms from my CGM. With this AP system, every day I woke up with glucose levels in range, so however my day started I was not chasing a high or low blood sugar.”
Looking back at her motives for volunteering, Wesner said: “Getting involved in AP trials to me was my chance to pay it forward for somebody else. I have lived 45 years with T1D, and I have lost friends to type one. And I think there’s an enormous responsibility that comes with being alive. I think there’s something comforting in knowing that my body was used for something that not only had the potential to make me healthier, but really was for other people. I do think there’s something to be said for doing good, feeling good, and this is what it felt like being part of trials.”
[8] Mary Tyler Moore had been diagnosed with T1D around 1970 at the age of 33. She soon became JDRF’s International Chairperson using her fame to raise awareness of the disease, increase private and federal research support, and testifying before Congress and inspiring others with the disease to live their lives fully and boldly
Chapter 13: The Finish Line
Spurred by the FDA guidance, goaded by the JDRF Animas deal9, the 800-pound gorilla finally come down from its mountain habitat and got into the game. Fittingly, it was FDA that played a key role in catalyzing the company’s involvement. “FDA would tell us flat out they were begging Medtronic, which was amazing, it was a 180-degree turn, like going from incredible headwinds at to having the wind at your back,” said Kowalski.
“In 2013 we reached out to Medtronic because they had a pump, a CGM, and an algorithm and we said we want to make this happen,” said Stayce Beck. “Let’s figure out what we need to do. So, we ended up meeting with Medtronic monthly to get them to accelerate. They originally weren’t planning on being in the market until 2019.”
In mid-2016, Medtronic announced that it had completed a trial of a hybrid closed loop system called the MiniMed 670G. The trial included 123 adolescents and adults at nine sites in the US and one site in Israel. It was a so-called pivotal trial, the last step in the drug and device development process before applying to the FDA for approval. Participants on the system for three months experienced a 44% reduction in time spent with a low blood sugar, a 40% reduction in dangerous low-blood-sugar territory, and an 11% decline with dangerous high blood sugars.
Just three months later, acting with unprecedented speed, on September 28, 2016, the FDA approved the 670G, and the Artificial pancreas Project had achieved its goal.
[9] In 2017, Animas exited the diabetes market without ever completing work on an AP. Speculation was that the company did not move quickly enough and was eclipsed by new iterations of the original Medtronic 670G.
Chapter 14: The Aftermath
Since the approval of the 670G, FDA has approved four additional systems, including the T:Slim X2 by Tandem, using the original algorithm developed by Boris Kovatchev, the Omnipod 5 by Insulet, using the algorithm developed years earlier by Frank Doyle’s team at UCSB, and Tandem’s Mobi, the Cam APS developed by Dr. Roman Hovorka’s team at the University of Cambridge in England, the iLet Bionic Pancreas that emerged from Ed Damiano’s team at BU10, and most recently, the Dean Kamen designed twiist from Sequel Med Tech. Thousands of others continue to use DIY systems.
As readers will recall, the landmark Diabetes Control and Complications Trial, or DCCT had found that people with T1D could reduce the risk of long-term complications by 35-76% through extremely tight blood-sugar control—keeping their levels in a range that largely eliminated dangerous highs and lows. But, as Brewer discovered when his son was diagnosed, meeting these ideal targets was nearly impossible with the technology in use in 2005.
Today, two decades after Brewer and Kowalski persuaded a skeptical JDRF to launch the AP Project, the latest available data leaves no doubt that their belief that developing AP systems would improve lives and reduce the risk of long-term health complications for people with T1D has been vindicated.
In June 2024, the T1D Exchange, a nonprofit dedicated to improving outcomes for people with T1D, completed a study11 of people living with T1D and found that children under 13, teens, and young adults between ages 18 and 29 using hybrid closed loop systems were achieving safe target blood-sugar levels 40-68% more often than those using insulin pumps and CGMs. Results were slightly lower for adults over 30 years old but still were statistically significant. The T1D Exchange data also showed that young people tended to have fewer severe low blood-sugar events on hybrid closed loops than those on pumps and CGMs.
“What we brought to bear is resulting in a safer and easier life for hundreds of thousands, and soon millions, of people with T1D, including my son, that is going to keep them safe until something like a cure comes along,” said Brewer.
[10] Work still continues on the elusive Goal Six multi-hormonal system.
[11] Automated Insulin delivery Use among 12,065 T1D Exchange Registry participants
Postscript
Pregnant women with T1D have a host of challenges and risks. They are eating for two, they have varying levels of stress, they have additional hormones impacting them systemically, including hormones that can upset blood-sugar control. In fact, one of two babies born to women with T1D have complications, most commonly preterm birth, large birth weight, and admission to neonatal ICUs. In 2008, Dr. Helen Murphy, a UK clinician who works with pregnant women with T1D, started collaborating with Roman Hovorka at the University of Cambridge, one of the early artificial pancreas research pioneers, who developed the CamAPS (artificial pancreas system) which was approved in the U.S. by FDA in May 2024.
Dr. Murphy’s focus was on using the AP systems to improve pregnancy outcomes for T1D Moms. Early systems were not sufficiently reliable to risk using with pregnant women, but by 2023 the time was ripe. Hovorka, Murphy, and colleagues around the UK conducted a trial of 124 pregnant women with T1D to test whether the Cambridge AP system could improve maternal glucose enough to benefit pregnancy outcomes. All users of this system experienced 10.5% more time in range throughout their pregnancies. Soon after, the UK’s National Institute of Health Care and Excellence issued clear guidance advising that all pregnant women in the UK be given access to pregnancy-specific AP systems. More than forty years after Bill Tamborlane asked Dean Kamen to develop a pump for his work with pregnant woman, the technology had become standard of care.
In February 2024, my daughter, diagnosed with T1D at age 14 in 2001, gave birth to her second child. She had her first in 2020. She wore the original DIY version of the Omnipod closed loop system in both pregnancies. Both babies were born healthy and on time.
“The loop system has truly transformed my life, making everyday activities easier and less stressful,” Emma said. “It allowed me to experience both of my “high-risk” pregnancies as “normal,” with tighter control over my blood-sugar levels so I could focus on enjoying the prenatal and postpartum journey. Day to day, this technology has given me greater stability and peace of mind, letting me live life fully without the constant worry of managing my diabetes.”
Alecia Wesner has been part of the Breakthrough T1D family since her diagnosis with type 1 diabetes (T1D) in Philadelphia in 1979. She has participated in Walks and Rides and served on her Community Board in New York City.
Since 2014, Alecia has participated in numerous T1D clinical trials, many of which were early artificial pancreas system trials. She has also participated in an implantable CGM clinical trial, a complication prevention trial, and numerous studies addressing the psychological and sociological impact of living with T1D.

Clinical trials are so empowering. Participating is a great way to contribute to science and also learn.”
Widespread awareness brings us closer to advancing breakthroughs to cure, prevent, and treat type 1 diabetes (T1D) until it is a condition of the past. Visit our Diabetes Awareness Month page to learn more about how you can get involved.
On Monday, Vertex made a monumental announcement. Their Phase 1/2 trial for VX-880 is converting to a Phase 1/2/3 pivotal trial, enrolling 50 total people.
It’s a huge first for type 1 diabetes (T1D). It’s the first time a scalable cure for some people with T1D is entering a Phase 3 clinical trial.
Let’s dive into what it means, how we got here, and what comes next.
What are cell therapies?
People with T1D are missing one big thing: the ability to produce insulin. In people with T1D, the immune system destroys the insulin-producing islets in the pancreas. If we can make insulin-producing cells and safely put them inside the body to replace the cells that were lost, we’ll have cured this disease.
Those are cell therapies—one of Breakthrough T1D’s biggest priorities, and what VX-880 is.
What is VX-880?
Vertex’s VX-880 is a stem cell-derived islet therapy. It uses stem cell-derived islets, which primarily contain beta cells, to restore the body’s ability to produce insulin. VX-880 requires the use of immunosuppressants to protect the transplanted cells from rejection.
The immunosuppressive regimen administered to accompany the infusion of cells is akin to those taken by recipients of solid tissue organ transplants. It has serious side effects. Finding alternative, less-toxic methods to prevent rejection is a key priority of Breakthrough T1D and next-generation products from Vertex.
The islets used in VX-880 derive from the Breakthrough T1D-funded work of Doug Melton, who first turned stem cells into insulin-producing cells in 2014.
VX-880 is not for the entire T1D community. It is only being tested in people with type 1 diabetes with severe hypoglycemia unawareness and significant hypoglycemic episodes. Vertex is testing it only in this patient population.
The data has been eye-opening
Vertex has regularly shared data on how this therapy is working in people, and it’s been incredibly exciting.
The most recent update came at the European Association for the Study of Diabetes annual meeting in September 2024. The data presented included:
- All participants who received the full dose of the therapy are showing benefits, including:
- Insulin production as measured by C-peptide
- (C-peptide is a biomarker that shows endogenous insulin production)
- Elimination of severe hypoglycemia
- Significantly improved glucose control, with HbA1c’s less than 7% and >70% time in range.
- Insulin production as measured by C-peptide
- 11 out of 12 participants have reduced or eliminated the need for external insulin.
- All 4 participants who received the full dose of cells with a follow-up after more than one year met the primary endpoint of eliminating severe hypoglycemic events and achieved the secondary endpoint of insulin independence.
- The safety profile is consistent with the immunosuppressive regimen, infusion procedure, and complications of longstanding T1D.
These data show that VX-880 can restore insulin production in people with type 1 diabetes. The primary endpoint for this study will be the proportion of study participants with insulin independence and absence of severe hypoglycemic episodes.
Stem cell versus deceased donor islets
Today, there is an FDA-approved islet transplant for individuals with T1D who are unable to achieve target HbA1c because of current repeated episodes of severe hypoglycemia called Lantidra. This therapy utilizes the Edmonton Protocol and relies on deceased-donor islets, which are taken from the pancreases of deceased organ donors. It can take up to three pancreases to harvest enough islets for one transplant.
There are simply not enough donor pancreases to gather enough islets for everyone with T1D. Stem cell-derived islets are the scalable solution to that problem.
These islets are manufactured in a laboratory and can be produced at scale. Vertex can make exponentially more cells in their facility than can be obtained via deceased donors—and they’ve broken ground on a manufacturing facility to make them.
What is a pivotal trial?
A therapy must undergo a series of clinical trials to assess its safety and efficacy before receiving FDA approval. The last stage is usually the Phase 3, or pivotal trial, which gathers the data required for an FDA submission.
This trial is a continuation of Vertex’s successful Phase 1/2 clinical trial.
Breakthrough T1D’s role
Breakthrough T1D has a collaborative partnership with Vertex and the leading researchers on the project, Drs. Doug Melton and Felicia Pagliuca, that goes back decades. Some key collaborations:
- Breakthrough T1D funded Doug Melton starting in 2004 to turn stem cells into insulin-producing cells. He accomplished this in 2014.
- In 2015, Melton founded Semma Therapeutics to turn these cells into curative therapies for T1D. In 2017, the T1D Fund made a catalytic investment in Semma.
- The T1D Fund also invested heavily in ViaCyte, which was acquired by Vertex in 2022.
Breakthrough T1D is thrilled this therapy is advancing. However, only a small subset of the T1D population will be authorized to use this therapy (assuming it gets FDA approval in the coming years). Our ultimate goal is a therapy that does not require the use of chronic immunosuppressants that the majority of people with T1D can use. (Vertex shares this goal).
We applaud Vertex for this achievement and we will continue our work to develop these strategies that will allow more people to use these potentially curative therapies.
Learn more about our work in cell therapies.
Other data shared
Vertex also shared that the Phase 1 trial for VX-264, their encapsulated stem cell-derived islet therapy, is progressing nicely and they expect to share data in 2025. Their gene-edited, hypoimmune islet therapy is still in development. These cells will not require the use of immune suppression.
What comes next?
Vertex has stated that its clinical trial is enrolling. We encourage anyone who is interested to visit clinicaltrials.gov and see if they are eligible to participate.
A novel immune therapy called tegoprubart made by Eledon Pharmaceuticals and being tested in kidney transplant patients has the potential to help beta cell transplants survive in people with type 1 diabetes (T1D) with fewer side effects. The first data from this Breakthrough T1D-funded study suggests it does. The study was presented at the 5th Annual International Pancreas and Islet Transplant Association/Harvard Stem Cell Institute/Breakthrough T1D Stem Cells Summit.
The need for a better drug regimen
Organ transplants are a wonder of modern medicine, but they require the use of immunosuppressive drugs. These drugs have serious side effects. They include but are not limited to an increased risk of infections and malignancies. They are also toxic to the kidneys, nerves, and islet cells themselves.
This is one reason that islet transplants, which follow the Edmonton Protocol, are only an option for people with hypoglycemia unawareness who have had severe hypoglycemic events. (Another reason is the extremely limited supply of deceased donor islets.) Today, Breakthrough T1D and researchers across various disciplines are working on developing novel and more targeted drugs. They goal: develop drugs that can inhibit the immune response to transplanted cells, like pancreatic islets, with more tolerable, or milder, side effects.
If the more serious side effects can be prevented, a much larger population of people with T1D could have access to this therapy. That is, assuming we have a larger supply of cells to transplant.
Enter tegoprubart
Tegoprubart is an immune therapy (anti-CD40 ligand) manufactured by Eledon Pharmaceuticals. It helps limit the body’s immune response.
It works by inhibiting CD-40L, a protein involved in the immune system. By blocking this protein, the drug interferes with how immune cells communicate with each other. It also increases the number of T regulatory cells, which suppress the body’s immune response.
This drug is currently in a phase 3 clinical trial in kidney transplants conducted by Eledon Pharmaceuticals. Results to date indicate that it has the potential to be a less toxic option to keep transplanted organs and cells, like islet cells, alive and healthy post-transplant.
Early results from new study are positive—and more data is needed
Breakthrough T1D is funding longtime collaborator Dr. Piotr Witkowski at the University of Chicago to use Eledon Pharmaceutical’s drug, tegroprubart, as an alternative to traditional immunosuppressives in people with T1D and severe hypoglycemia who have received deceased donor islet transplants.
Per Eledon Pharmaceutical’s press release:
- The first two out of three subjects treated with tegoprubart as part of an immunosuppression regimen to prevent transplant rejection achieved insulin independence. They remained insulin-free, with glucose control in the normal range.
- The third subject was recently transplanted. They reduced insulin dosage by 60% within three days post-transplant.
- Islet engraftment in the first two subjects with tegoprubart was three to five times higher than engraftment in three subjects receiving standard-of-care tacrolimus-based immunosuppression.
- Treatment with tegoprubart was well tolerated.
This news is exciting and has the potential to be life-changing for people with T1D. Removing the 24/7, 365-day-a-year burden of T1D without toxic side effects through curative therapies is our goal.
However, the data is only in two people to date. We look forward to seeing data from more transplants in the future.
How this fits into our strategy for cures
Breakthrough T1D is laser-focused on cell therapies, which implant insulin-producing cells into the bodies of people with T1D. Our ultimate goal is an unlimited, or allogenic, supply of cells derived from stem cells. These cells can be transplanted into anyone living with T1D without the use of chronic immunosuppressives.
In other words, we need two things: a unlimited cell source and a non-toxic way to keep the cells healthy.
Incredible recent progress has been made toward both of these goals. For example, in Vertex’s VX-880 study, they are using stem-cell-derived islets, and the 11/12 recipients are fully insulin-independent after 1 year. Individuals in this study are on the traditional post-transplant immunosuppressive regimen. (The study is ongoing and has expanded enrollment to 37 people.)
Breakthrough T1D, Vertex, and others are also working on how to keep the cells safe using a combination of encapsulation, gene editing, and other tactics. Several of these studies are in clinical trials.
Tegoprubart can potentially be a viable tactic to keep these cells functional durably. That’s why we’re funding the study along with the Cure Alliance. It’s also why the T1D Fund invested in Eledon Pharmaceuticals several months ago and again on October 29.
What we’re saying
“Breakthrough T1D is proud to fund and support this research and is encouraged by the tegoprubart study showing that patients who received islet transplants with a tacrolimus-free immunosuppressive regimen are making insulin again,” said Breakthrough T1D Chief Scientific Officer Sanjoy Dutta, Ph.D. “Islet replacement therapies are a key priority for Breakthrough T1D, and we’re committed to driving research that moves us toward a world where these therapies are available to the broader T1D community. Achieving this requires novel approaches to keep transplanted cells functional with a tolerable immunosuppression regimen. These results are an important step toward that goal, and we look forward to seeing additional data.”
Read more in the press release.
At the 2024 International Society for Pediatric and Adolescent Diabetes (ISPAD) Conference in Lisbon, Portugal, the world’s leading diabetes researchers, academics, and members of industry gathered to share the latest and greatest in diabetes research. Breakthrough T1D leaders and many of our funded researchers and collaborators were on hand —for ISPAD’s 50th birthday—to share new science, breakthroughs, and a glimpse at what the next generation of T1D therapies will look like.
Let’s look at a few highlights!
The T1D Index Levels Up

Breakthrough T1D Chief Scientific Officer Sanjoy Dutta, Ph.D., and Thomas Danne, MD, Chief Medical Officer, International presented a much-awaited update on the T1D Index at ISPAD.
The T1D Index is a first-of-its-kind data simulation tool that measures the human and public health impact of the T1D crisis in every country across the globe. Until the creation of the Index, there have been wide gaps in the data about the incidence and impact of T1D. Leveraging data and insights from the T1D Index can help change the lives of people living with T1D by identifying attainable country-by-country interventions, including timely diagnosis, accessible care, and funding research that could lead to cures.
First , Dr. Dutta gave a refresher on our recent brand evolution, which includes why this change was necessary and how our research strategy has not changed.
Dr. Danne followed with a reintroduction of the Index, its place in our mission, and how we improved it. This includes:
- An enhanced dashboard and user interface designed to improve user experience
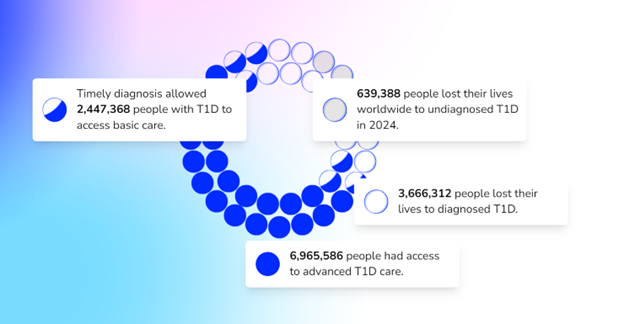
- A plan to develop Index 2.0
- An invitation and plea for all countries and existing registries to collaborate with data to enhance and develop Index 2.0
- Advanced simulation capabilities allowing for scenario analysis.
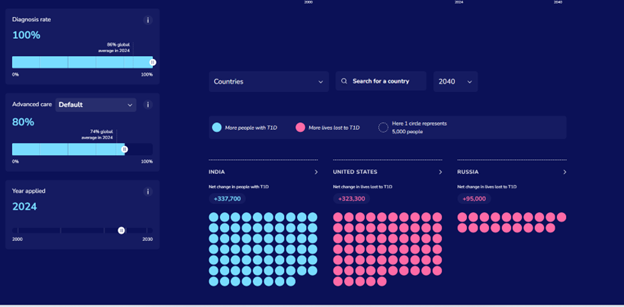
- Validation of data against available national registry data.
- Performance upgrades, including improved platform speed and minor adjustments to further refine accuracy.
In the future, the Index aims to have even more detailed data on a regional level.
Key takeaway:
Breakthrough T1D is focused on improving the lives of people with T1D all around the world—that includes every country in the world. The Index is key to defining the global scope of T1D and the country-by-country solutions to bring better outcomes to everyone affected by T1D.
Is metabolic control enough for young people with T1D?
We have known for decades that T1D is accompanied by the risk of developing complications. The conditions that are most associated with T1D include diabetic eye disease (retinopathy), kidney disease (nephropathy), and heart disease (cardiovascular disease). This is not an exhaustive list—there are many others, including mental health disorders. These complications most commonly occur in adults living with T1D, but new research suggests there’s more we can do to monitor and intervene with these complications in youths with T1D in addition to striving for ideal glycemic control.
At ISPAD, Breakthrough T1D-funded researcher Risa Wolf, MD, discussed long-term microvascular and macrovascular complications in T1D, including the complications mentioned above, and the data is striking. There is a low percentage of complications 4-7 years after diagnosis but after 12 years:
- 24% will have a decline in eGFR (a biomarker for kidney function)
- 13% will have neuropathy
- 52% will have retinopathy
- 28% will have dyslipidemia (abnormal levels of lipids in the blood)
Thankfully, leaps in care management, including automated insulin delivery systems and continuous glucose monitors, have led to improvements in A1c and, in turn, the rate at which people develop complications. But more can be done.
Through regular screening for these more common complications for all youths with T1D, we can better identify, diagnose, and monitor individuals as they develop these complications—especially because many of these screenings are low-cost.
Breakthrough T1D, for example, is funding Dr. Wolf to use the autonomous AI system (IDx-DR, Digital Diagnostics), which is FDA-approved for adults 21+ to detect and diagnose diabetic retinopathy in children. Ideally, this will lead to FDA approval and a better tool for monitoring this complication in kids.
Drs. M. Loredana Marcovecchio – another Breakthrough T1D funded investigator and Didac Mauricio took a similar look at vascular complications. Their presentations had related themes—it is critical to develop ways to identify complications like cardiovascular disease earlier. If they’re identified earlier, individuals can take advantage of the approved therapies.
Key Takeaway:
Like most areas of T1D management: there are still significant unmet needs.
We need to have a long view of these complications and their pathogenesis, which can start in youth. Glycemic control is critical to preventing the onset of these complications, but it is not always enough. Cardiovascular disease, retinopathy, dyslipidemia, and more can all be detected at a young age, which gives more time for interventions.
Breakthrough T1D is working on all of this in our Improving Lives program.
ISPAD / Breakthrough T1D Sessions
Breakthrough T1D Chief Scientific Officer Sanjoy Dutta, Ph.D. joined several collaborators for two sessions.
In the first, titled “Joint-Symposium Breakthrough T1D (formerly JDRF)-ISPAD: Type 1 Diabetes (T1D) Treatments: Hope and Promise Ahead,” two recipients of ISPAD/Breakthrough T1D fellowships, which allow care providers to travel to and receive training at world-class diabetes centers, shared their experiences and how the fellowship enabled them to improve the care they can provide to their communities.
After a presentation from Chantal Matthieu, a longtime Breakthrough T1D investigator, partner and reviewer, Dr. Dutta presented an overview of our work, our vision, and how the next generation of researchers in the room can apply for funding and join us in our work.
In the second session, the Joint ISPAD – Breakthrough T1D symposium, Dr. Dutta and others provided an update on our progress in treating this disease and what’s on the horizon. They touched on the many ways we can slow down disease progression, the exciting state of stem cell-derived cell therapies (they’re in human clinical trials and they’re producing insulin), and how artificial intelligence can play a role in reducing or eliminating T1D misdiagnosis in adults which can be as high as 40%.
Key takeaway:
It’s an exciting time in T1D research, and Breakthrough T1D’s strategy has mapped out what it takes to get to cures—including the importance of bringing in the next generation of researchers to contribute.
Revolutionizing Diabetes Care: Cutting-Edge Therapies
This session, which was a personal favorite of the author, gave updates on the biggest priorities we have at Breakthrough T1D: cures and how close we are to realizing them.
Spoiler alert: we’re not that far away.
It featured presentations from several Breakthrough T1D-funded researchers, including Colin Dayan, MD, Lori Laffel, MD, and Kimberly Simmons, MD.
First up: Dr. Simmons spoke about Tzield, the first disease-modifying therapy approved for T1D, and its use in the real world. A quick summary of the data:
- 322 people have received the drug to date
- The side effects reported in the clinical trial are consistent with what has been seen at the Barbara Davis center
- “Most of our patients have told us that they would do this treatment again.”
- Long term data for how people are tolerating the therapy and progression to T1D is not available yet.
As a reminder, Tzield was FDA-approved in November 2022 for individuals with stage 2 T1D to delay onset by an average of two years. Learn more in this comprehensive story on how it came to be and the role Breakthrough T1D played in getting it to market.
Dr. Michael Rickels then explored cell replacement therapies: where we are and where we’re going. His presentation covered the current landscape: We can use cadaveric islets to restore insulin production in people with T1D. However, the requirement for chronic immunosuppression and scarcity of donor islets mean this is not a viable option for the vast majority of people with T1D.
The next step is using an unlimited supply of stem cell-derived islets and discovering methods to keep them safe without the use of chronic immunosuppression, which is consistent with our strategy in this area.
Dr. Rickels also touched on the study that used autologous cells to restore insulin production in a person with T1D.
Lastly, Dr. Colin Dayan gave an update on combination therapies to preserve beta cell function in people with T1D.
Combination therapies utilize several different therapies that work by discrete mechanisms to target a singular problem. An example is tuberculosis or HIV, which both relied on complicated treatments composed of different pills that had to be taken at different times of day but are now treated with singular pills.
As we have shown, there are many drugs that can preserve insulin production. Now we’re learning which drugs can be taken together to preserve that insulin production longer. Dr. Dayan discussed the T1D Plus study (which builds on the Ver-A-T1D trial), which is funded by Breakthrough T1D, JDRF Australia, INNODIA, and the Helmsley Charitable Trust. This study will test several different drug combinations to see their effectiveness, including Tzield, verapamil, antithymocyte globulin (ATG), and golimumab.
Not only will this trial test multiple drugs—the innovative design of the study will speed up the length of the study, shaving years of the time it will take to see what combinations work best.
Key takeaway:
Breakthrough T1D is at the center of cell therapies and disease-modifying therapies. The clinical trials being run in humans today are very exciting and are a glimpse into how this disease could be treated in the not-too-distant future. It’s possible there will be approved combination therapies to slow progression and cell replacement therapies that restore insulin production entirely in the not-too-distant future.
Until next year!
These are just a few highlights from three days of sessions and presentations that cover our entire research portfolio. For example, Dr. Danne presented on the importance of early detection due to its numerous, clearly defined benefits and strategies for identifying these youths with stage 1 and stage 2 T1D. There were also sessions on the impact of AID systems, specific areas of complications, and much, much more.
We’re already looking forward to next year!
Breakthrough T1D joined thousands within the diabetes community at the American Diabetes Association (ADA) 84th Scientific Sessions. Held June 21–24 in Orlando, FL, scientists presented 190+ studies with Breakthrough T1D funding—at present or in the past—to encompass breakthrough clinical trials and significant research studies that are paving the way to novel treatments and technologies for T1D.
Here is Aaron J. Kowalski, Ph.D., CEO of Breakthrough T1D, with the key takeaways from the conference and below is a written summary of Breakthrough T1D highlights.
Cures
Stem cell-derived islet replacement therapy: Update on Vertex’s VX-880
Breakthrough T1D research area: Cell therapies
Cell replacement therapies, including stem cell-derived islet therapy, were on fire, again, with Vertex Pharmaceuticals once more leading the way in clinical trials. Vertex launched its clinical trial of VX-880, a stem cell-derived islet therapy in T1D for individuals with low blood sugar (called hypoglycemia) unawareness, in combination with immunosuppressive therapy to protect the cells from rejection, in the summer of 2021. Within Parts B and C, 12 participants have received the full, target dose of cells, and the results are remarkable. 11 of the 12 participants have reduced or eliminated the need for external insulin. What’s more, all 3 individuals who received the therapy at least 12 months ago have eliminated hypoglycemic events and are fully off external insulin—meeting the primary and secondary endpoints. There were no significant side effects related to VX-880, and the trial has expanded to enroll 20 more participants, to 37.
In summary: VX-880 has curative potential, with achieving insulin independence, eliminating severe low blood-sugar events, and significantly improving blood-sugar control all within reach.
Breakthrough T1D leadership: Vertex’s phase I/II clinical trial of VX-880 was pioneered by Doug Melton, Ph.D., whose years of Breakthrough T1D-funded research led to successfully transforming stem cells into beta cells in 2014, and a catalytic investment from the T1D Fund: A Breakthrough T1D Venture in Semma Therapeutics—a biotech company founded by Melton to develop a stem cell-derived islet therapy for T1D, which was acquired by Vertex Pharmaceuticals.
Tzield® (teplizumab-mzwv): Additional improvement in Stage 3 T1D
Breakthrough T1D research area: Disease-modifying therapies
Kevan Herold, M.D., presented a secondary analysis of the phase III PROTECT clinical trial. In October 2023, Tzield® (teplizumab-mzwv) showed that it can slow the loss of beta cells and preserve beta cell function in newly diagnosed (stage 3 T1D) children and adolescents ages 8-17. In the present analysis, Tzield® demonstrated that, in addition to slowing down the loss of beta cells in new-onset T1D, it could decrease insulin dose, improve time-in-range, and decrease severe low blood sugar events. This reinforces our commitment to supporting therapies that preserve beta cells at onset, which is important for the prevention of complications and improvement in new-onset clinical factors.
Breakthrough T1D leadership: Dr. Herold has been supported by Breakthrough T1D since the late 1980s. In his research, he showed that he could prevent autoimmune diabetes with an immune-modifying antibody (which later became a humanized version) and was the lead on the clinical trial that demonstrated that Tzield could delay the onset of T1D in people almost certain to develop the disease. In November 2022, Tzield was approved by the FDA to delay the onset of the disease (Stage 3) in at-risk (Stage 2) individuals ages 8+.
First international consensus monitoring guidance
Breakthrough T1D research area: Early detection
Breakthrough T1D spearheaded the first internationally agreed-upon monitoring guidance for anyone who tests positive for T1D autoantibodies. These provide guidelines for monitoring children, adolescents, and adults who test positive, along with recommended monitoring frequencies and actions for healthcare professionals when the risk of progression toward symptomatic T1D is high. The guidance also includes recommendations for educational and psychosocial support for positive T1D antibody individuals, including their families and caregivers.
2+ T1D-related autoantibodies—antibodies that are directed toward your own body—means you have an almost 100% chance of developing T1D in your lifetime.
Breakthrough T1D leadership: Until now, there were no monitoring guidelines for individuals who tested positive for T1D autoantibodies. But, for the first time, individuals, families, and healthcare professionals have concrete next steps to monitor early-stage T1D progression and catch symptoms early to prevent DKA. This will reduce DKA at diagnosis and identify autoantibody-positive individuals to take part in preventive treatment or clinical trials. This is a landmark publication.
Diabetic ketoacidosis (DKA) is a life-threatening complication, typically due to a shortage of insulin in the body, causing symptoms like dehydration, nausea and vomiting, confusion, and difficulty breathing. Approximately one-third of people in the U.S. present with DKA at the time of T1D diagnosis.
Improving lives
AID technology updates
Breakthrough T1D research area: Artificial pancreas
A ton of presentations focused on the artificial pancreas, or automated insulin delivery (AID), systems. The Medtronic 780G was tested in high-risk youth with T1D, with 80 participants aged 7-25 years; 75% of whom face economic deprivation. Participants saw an average HbA1c reduction of 2.5% (from an average baseline HbA1c of 10.5% to 8%), improvement in time-in-range, and a reduction in low blood sugar events, and there were no incidences of DKA or severe hypo in the AID group. Tandem Mobi had high rates of satisfaction with the system, with the vast majority of respondents (86%) saying they were satisfied or very satisfied. Additionally, 86% of respondents agreed or strongly agreed that Tandem Mobi improved their quality of life. These indicate high real-world satisfaction with Tandem Mobi among early pediatric and adult adopters. Sequel Med Tech twiist AID system, which was FDA-cleared for people with T1D aged 2+ in March 2024, uses the DEKA Loop algorithm, which is based on the Breakthrough T1D-funded FDA-approved Tidepool Loop. This twiist system is expected to launch at the end of 2024.
Breakthrough T1D leadership: In 2005, Breakthrough T1D launched the Artificial Pancreas Project, a multi-million-dollar, multi-year initiative to accelerate the development of systems for automated blood sugar control. The Artificial Pancreas Project would go on to fund more than 150 grants, including 50+ clinical trials, funded by Breakthrough T1D and backed by the Breakthrough T1D Artificial Pancreas Consortium, to make the artificial pancreas system a reality.
“I think [Breakthrough T1D] got us here a lot faster than we could have any other way.”
Francine R. Kaufman, M.D., Chief Medical Officer, Senseonics
Former CMO and Vice President, Medtronic Diabetes (2012-2018), who launched the first artificial pancreas system in 2016
ADA Recipient, 2024 Lois Jovanovic Transformative Woman in Diabetes Award
GLP-1 treatments: Weight loss for T1D?
Breakthrough T1D research area: Glucose control
There is increasing interest in the off-label use of glucagon-like peptide 1 (GLP-1) treatments for weight loss in people with T1D, given that more than two-thirds of people with T1D in the U.S. are overweight or obese. Presented at ADA, the T1D Exchange survey data found that almost 7% of individuals with T1D reported using a GLP-1 therapy, with 65% of people using semaglutide (Ozempic® and Rybelsus® for T2D and Wegovy® for weight loss) and 17% of people using tirzepatide (Mounjaro® for T2D and Zepbound™ for weight loss). To verify the effectiveness and safety profile of this drug class in people with T1D, a retrospective study and a prospective study were presented at ADA. In a retrospective study, those on tirzepatide had 21% of weight loss over one year, with significant blood-sugar improvements. A prospective clinical trial of GLP-1 treatments (semaglutide or tirzepatide) in overweight or obese individuals with T1D showed that it could lead to significant improvements on body weight and HbA1c.
Breakthrough T1D leadership: Breakthrough T1D funded copious research in the 1980s through today to understand the role of glucagon and GLP-1 in T1D. Work revealed that GLP-1 encourages the release of insulin from the pancreas and reduces the release of glucagon, and clinical trials demonstrated that it was effective in treating T2D, eventually leading to its approval in 2005. There are now seven GLP-1 medicines on the market
Correlation between time-in-range and diabetic eye disease
Breakthrough T1D research area: Complications
Using continuous glucose monitoring (CGM) data, the Virtual DCCT Project successfully reproduced HbA1c outcomes in the original DCCT trial. The study found that decreased time-in-range was associated with increased risk for diabetic eye disease. This study reflects continued momentum to validate time-in-range as a primary endpoint in clinical trials.
The Diabetes Control and Complications Trial (DCCT) was a major clinical study involving almost 1,500 participants, ages 13-39, with T1D, conducted from 1983 to 1993. The study compared the effects of standard vs. intensive control of blood sugar on the complications of diabetes. The results showed that intensive blood sugar control reduces the risk of eye disease by 76%, kidney disease by 50%, and nerve disease by 60%.
Breakthrough T1D leadership: Breakthrough T1D has supported eye disease research since its beginning, and has driven discoveries that have reduced the risk of blindness by 95%, including laser therapy and anti-VEGF treatments.
SGLT inhibitors in adolescents with T1D
Breakthrough T1D research area: Complications
In the ATTEMPT (Adolescent T1D Treatment with SGLT2i for hyperglycEMia & hyperfilTration) trial, researchers evaluated the impact of the SGLT inhibitor, dapagliflozin (Farxiga®/Forxiga®), versus a placebo, in combination with insulin therapy, in adolescents with T1D, to find if it can improve kidney function. Findings from the Breakthrough T1D-funded clinical trial showed that a low-dose SGLT inhibitor could improve kidney function and blood-sugar management. There were no significant differences in adverse events, elevated ketone levels, and low blood sugar events. Overall, these results corroborate findings from previous studies in the adult population, which have demonstrated that there are positive kidney effects of SGLT inhibitors, affecting many other pathways to confer multiple benefits.
Breakthrough T1D leadership: In addition to eye disease research, Breakthrough T1D has supported kidney disease research from the beginning. We evaluated the role of puberty in diabetic kidney disease in the early 1990s and found that it played a major role in the development of diabetic kidney disease, where pubertal changes led to increased insulin resistance, rapid kidney growth, and increasing numbers of people being overweight or obese.
Psychosocial screening tools
Breakthrough T1D research area: Behavioral health
There are many ways that T1D can affect people’s social, mental, and emotional well-being, known collectively as psychosocial health, and many Breakthrough T1D-funded researchers presented on psychosocial issues, including Jessie Wong, Ph.D., Holly O’Donnell, Ph.D., and, in place of Jeffrey Gonzalez, Ph.D., Elizabeth Beverly, Ph.D. In addition to diabetes distress, T1D brings about an increased risk of depression, anxiety, executive function, and other psychosocial challenges. Fortunately, there are screening tools to identify who needs help, of varying reliability and validity. But the conclusion: Increase the number of qualified behavioral health professionals with training in T1D.
Breakthrough T1D leadership: Since the Breakthrough T1D Psychosocial Health Program was started in 2018, we have trained 24 psychologists, who have developed expertise in addressing the unique needs of people impacted by T1D and awarded grants of more than $30 million to address the psychosocial challenges of the condition.
You can view all of the oral and poster presentations on the Diabetes journal website.